 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Voluntary HIV Testing as Part of Routine Medical Care --- Massachusetts, 2002In 2003, CDC released Advancing HIV Prevention: New Strategies for a Changing Epidemic. One of the four strategies of this initiative is to expand routine, voluntary human immunodeficiency virus (HIV) testing (1). This report describes the results of a state-funded program in Massachusetts that offered HIV counseling, testing, and referral (HIV CTR) to patients entering one of four hospital-associated urgent care centers. Among the 3,068 patients tested, the program identified an HIV seroprevalence of 2.0%. The findings underscore the effectiveness of routine HIV CTR in HIV case identification. The Massachusetts Department of Public Health (MDPH) AIDS Bureau identified the 15 cities in Massachusetts with the highest HIV prevalence. On the basis of patient volume and existing HIV primary care services, four hospital-associated urgent care centers in these cities were selected for program implementation. The program, called "Think HIV," was designed to assist centers in routine HIV counseling and testing, facilitate patient follow-up for test results, and promote strategies for linkage to care. Patient privacy and the availability of adequate, expedient HIV care for those who tested positive were essential components of the program. After registration for urgent care, patients were offered the opportunity to speak with a "health educator," a certified counselor with case-management experience trained specifically in sexually transmitted diseases, hepatitis C, and HIV. Counselors were available weekdays and some weekends. Patients who agreed to speak with a health educator were told that voluntary, confidential HIV CTR was now offered routinely to urgent care patients. Patients who declined to speak with a health educator were asked about their reasons for refusal, and those who reported they were already known to be HIV-infected were asked if they were receiving HIV care; if not, they were linked to care. Upon completion of counseling, confidential HIV tests were performed by using the oral swab, OraSure® HIV-1 antibody detection system (Epitope, Inc., Bethlehem, Pennsylvania). Patients were instructed to return to the urgent care center for test results 14 days later, when results were provided and post-test counseling was performed. Substantial efforts, including a minimum of four telephone calls and a follow-up letter, were made to locate all patients testing negative or positive who did not return for results. Additional efforts, including offering transportation vouchers and contacting homeless shelters, were made for persons testing positive who failed to return. At each center, an HIV intake nurse from an HIV outpatient clinic provided assistance to patients during posttest counseling, arranged follow-up HIV clinical care appointments, and often brought patients to their care appointments. During 2002, the first year of the program, 10,352 patients were offered HIV counseling at the four centers, accounting for approximately 10%--15% of all patients entering these urgent care centers and a percentage determined by counselor capacity. Of the 10,352 patients offered HIV testing, 7,071 (68%) declined testing; 6,291 (89%) of these 7,071 were willing to answer inquiries about their refusal to undergo testing. The reasons given for testing refusal included one or more of the following: 1) did not feel at risk for HIV (2,974 [47%]), 2) tested for HIV before (2,624 [42%]), 3) felt too ill (686 [11%]), 4) testing takes too long (281 [4%]), 5) information too personal (120 [2%]), and 6) already known to be HIV-infected (86 [1%]). Of the 2,573 patients reporting previous HIV testing who also provided the dates of the test, 1,542 (60%) reported their tests were performed in 2002 (Table). Among the 3,068 patients with completed test results, 60 were HIV-infected (HIV prevalence: 2.0%); of these, 49 (82%) returned for their results. Of the first 42 patients for whom linkage-to-care data were available, all 42 had at least one documented follow-up visit for HIV care. During the interview process, the program also identified six additional patients who reported they were known to be HIV-infected and who described themselves as either not having a doctor or not being in care. These patients were referred for follow-up HIV care. Four of these six patients had confirmed attendance at their first HIV care appointment. The program was funded by the MDPH AIDS Bureau. Overall, the cost of the program for the first 12 months was $349,400, which amounted to $7,100 for each of the 49 new HIV-infected patients told of their diagnosis or $5,800 for each of the 60 new cases identified. Reported by: RP Walensky, MD, Massachusetts General Hospital; KA Freedberg, MD, Harvard Medical School; E Losina, PhD, Boston Univ School of Public Health; PR Skolnik, MD, JM Hall, Boston Univ Medical Center; L Malatesta, MPH, GE Barton, CA O'Connor, MSN, JF McGuire, PhD, AIDS Bur, Massachusetts Dept of Public Health. Editorial Note:This report describes results of the Think HIV program in Massachusetts, which offered voluntary HIV CTR routinely to patients entering four urgent care centers. Because these centers did not previously have routine HIV CTR available, the majority of the 60 newly identified HIV patients likely would not have been identified until later in the course of their disease without the program. Health-care providers often discourage HIV testing in urgent care centers because of concerns regarding adequate training, pre- and post test counseling, and follow-up for patients testing HIV positive (2). Because many medically underserved patients at high risk for HIV use urgent care centers and emergency departments for their primary care, repeated opportunities for HIV diagnosis in these patients often are missed (3). Simply making a diagnosis of HIV, however, does not ensure the individual and public health benefits of HIV care. Previous reports have indicated that a mean delay of entry into HIV care of 3 months occurs after HIV diagnosis, with 32% of patients delaying >2 years and 18% delaying >5 years (4). To combat this lag to care, the program emphasized a formal linkage-to-care mechanism. An identified intake nurse at each center confirmed that newly HIV-diagnosed patients had rapid, immediate communication with members of their future health-care team. Success with the linkage component of the program is evidenced by a first appointment attendance rate of 100%, compared with 34% in another urgent care routine testing program in Atlanta (5). Results from CDC's Antiretroviral Treatment and Access Study also demonstrated substantial improvements in entry into HIV care with the presence of HIV case-management personnel. Patients who had two to three visits with a case manager during a 3-month period attended more HIV care visits, compared with patients who did not have these encounters (6). HIV testing as part of routine care has been delegated to primary care providers. In a 10- or 15-minute provider visit intended to cover many components of medical care, HIV CTR typically is not performed. By using counselors committed to this effort, the program had an estimated cost per new HIV patient identified of <$6,000, a figure that would be reduced with more streamlined pretest procedures of providing information about HIV testing (as recommended in CDC's Advancing HIV Prevention initiative) rather than the previously recommended extensive pretest counseling (1). Model-based cost-effectiveness analyses of routine HIV screening in primary care, outpatient, and inpatient settings have projected cost-effectiveness ratios of $22,000--$36,700 per quality-adjusted life year gained, which is more cost-effective than screening for colon cancer (7--10). The findings in this report are subject to at least two limitations. First, although efforts were made to test all patients entering the urgent care centers, access to HIV testing was based on counselor availability. Second, centers with suspected high HIV prevalence were chosen, and results should not be generalized to all urgent care centers throughout the United States. CDC's initiative Advancing HIV Prevention: New Strategies for a Changing Epidemic calls for including HIV testing as a routine part of medical care to increase the number of HIV-infected persons who are aware of their positive serostatus (1). The diagnosis of HIV in HIV-infected persons is a priority in the United States. Routine, voluntary HIV screening programs in urgent care centers in areas of high HIV prevalence are feasible and can be successful at diagnosing persons with HIV and linking them to appropriate HIV care. CDC is currently funding such projects in out-patient care clinics and emergency departments in four states. In addition, CDC will be funding community-based organizations and health departments to assist with linkage and referrals in facilities in areas of high HIV prevalence and will evaluate the cost-effectiveness of this strategy. References
Acknowledgments This report is based in part on contributions by HE Smith, Massachusetts General Hospital, the hospital staff, urgent care center staff, and HIV counselors at Boston Medical Center, Baystate Medical Center, Univ of Massachusetts Medical Center, Cambridge Hospital, Whidden Hospital, Boston; AIDS Bur, Partners/Fenway/Shattuck Center for AIDS Research, Massachusetts Dept of Public Health. National Institute of Allergy and Infectious Diseases, National Institute of Mental Health, National Institutes of Health.
Table 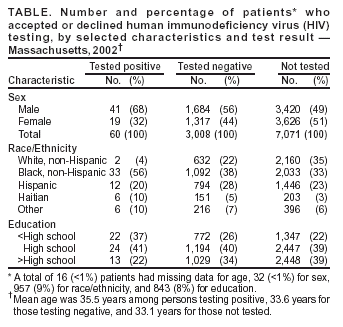 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 6/23/2004 |
|||||||||
This page last reviewed 6/23/2004
|