 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Genetic Testing for Breast and Ovarian Cancer Susceptibility: Evaluating Direct-to-Consumer Marketing --- Atlanta, Denver, Raleigh-Durham, and Seattle, 2003Breast and ovarian cancer are the second and fifth leading causes of cancer death, respectively, among women in the United States (1). One in eight women will have breast cancer during their lifetimes, and one in 70 will have ovarian cancer. Mutations in two genes, BRCA1 and BRCA2 (BRCA1/2), are associated with predisposition for inherited breast and ovarian cancer and are identified in 5%--10% of women with breast or ovarian cancer (BOC) (2). Since 1996, genetic testing for these mutations has been available clinically (3); however, population-based screening is not recommended because of the complexity of test interpretation and limited data on clinical validity and utility (1,4--6). Despite the test's limited applicability in the general population, the U.S. provider of clinical BRCA1/2 testing (Myriad Genetic Laboratories, Inc., Salt Lake City, Utah) conducted a pilot direct-to-consumer (DTC) marketing campaign in two cities (Atlanta, Georgia, and Denver, Colorado) during September 2002--February 2003. Although DTC advertisements have been used to raise consumer awareness about pharmaceuticals (7), this was the first time an established genetic test was marketed to the public. To assess the impact of the campaign on consumer behaviors and health-care provider practices, CDC and the respective state health departments for the pilot cities and two comparison cities (Raleigh-Durham, North Carolina, and Seattle, Washington) surveyed consumers and providers. This report summarizes results of those surveys, which indicated that consumer and provider awareness of BRCA1/2 testing increased in the pilot cities and that providers in these cities perceived an impact on their practice (e.g., more questions asked about testing, more BRCA1/2 tests requested, and more tests ordered). However, in all four cities, providers often lacked knowledge to advise patients about inherited BOC and testing. These findings underscore the need for evidence-based recommendations on appropriate use of genetic tests and education of providers and the public to achieve maximum individual and public health benefit from genetic testing. Women aged 25--54 years with personal or family histories of BOC and their health-care providers were target audiences of the DTC campaign. The campaign consisted of television, radio, and print advertising to raise awareness about BRCA1/2 testing and to motivate women to ask their providers how genetic testing might help assess BOC risk and guide them to effective medical management options. Providers received precampaign information and patient support materials (8). During April 21--May 20, 2003, a 51-question consumer telephone survey was conducted by using randomly generated household telephone numbers. Approximately 1,600 women were targeted for participation. Survey questions addressed family history, campaign awareness, interest in BRCA1/2 testing, cancer concerns, and interactions with health-care providers, family members, and friends. On May 1, 2003, providers were mailed a 35-question survey and a monetary incentive. Questions surveyed knowledge of inherited BRCA1/2 mutations, campaign awareness, and perceived changes in practice subsequent to the campaign. Approximately 1,600 physicians were selected randomly from the American Medical Association master list to be proportionally representative of four specialties (i.e., family practice, internal medicine, obstetrics/gynecology, and oncology). Consumer SurveyA total of 1,635 women completed the survey (participation rate: 45%); the majority (79%) were non-Hispanic white, with a median age of 40 years and more than a high school education (75%). Thirteen percent had a family history of BOC in a first-degree relative (e.g., parent or sibling). In the pilot cities, consumers were substantially more likely than those in the comparison cities to have heard of the test and to have seen a television, radio, or magazine advertisement; however, perceived knowledge about testing did not differ between consumers in the pilot and comparison cities (Table 1). No differences were observed between pilot and comparison cities in the percentage of women who reported talking to anyone about the test (Table 1) or in the level of concern about their risk for BOC. Among women who had heard of the test, interest in testing did not vary by city (Table 1). Among women who had heard of and were interested in the test, 20% had a first-degree relative with BOC, compared with 17% of women who had heard of the test but were not interested. Provider SurveyA total of 1,054 providers completed the survey (participation rate: 66%); the majority (66%) were male, had been in practice for >10 years (62%), and evaluated <100 patients per week (65%). In the pilot cities, providers were more likely than those in comparison cities to report that they and their patients saw or heard an advertisement about genetic testing for BOC (Table 2). When asked to compare the previous 6 months with the same period 1 year before, more providers in the pilot cities than in comparison cities reported an increase in the number of patients who had asked questions about testing, asked for genetic counseling referrals to consider testing, and requested testing. Providers in the pilot cities also reported ordering more tests but not more referrals to genetics or oncology centers (Table 2). Provider knowledge did not differ between the pilot and comparison cities. Fifty-two percent of providers were aware that a BRCA1/2 mutation can be inherited from either parent, and 46% knew that a woman with a sister with a known BRCA1 mutation has a 50% risk for inheriting the same mutation. Oncologists and obstetricians/gynecologists were more likely to answer knowledge questions correctly. The majority of providers believed that learning more about genetic testing for BOC risk was relevant to their practice. Reported by: J Jacobellis, PhD, Colorado Dept of Public Health and Environment. L Martin, MS, Georgia Dept of Human Resources. J Engel, MD, North Carolina Div of Public Health. J VanEenwyk, PhD, Washington State Dept of Health. LA Bradley, PhD, Office of Genomics and Disease Prevention, Office of the Director; S Kassim, MD, C Jorgensen, DrPH, Div of Cancer Prevention and Control, National Center for Chronic Disease Prevention and Health Promotion; JA Litch, MD, MF Myers, PhD, EIS officers, CDC. Editorial Note:The findings from the provider survey indicate that DTC advertisements might have motivated women interested in learning more about BRCA1/2 testing to talk to their physicians and request testing. Findings from the consumer survey suggest that women in the pilot cities were more aware of BRCA1/2 testing than those in the comparison cities. No evidence suggested an increased interest in the test among women most suited for BRCA1/2 testing (i.e., those having a first-degree relative with BOC). The demonstrated lack of provider knowledge underscores the need for additional education; providers in certain specialties were unprepared to address the complexities surrounding genetic testing for susceptibility to BOC. Complexities of the BRCA1/2 test have been described (2,4,5). Most BOC is not associated with BRCA1/2 mutations. Testing has been directed at women with a family history of cancer; however, even in this group, test interpretation is complex, and genetic counseling is recommended (4--6). Women with an identified BRCA1/2 mutation have a substantially increased lifetime risk for BOC, but morbidity and mortality might be reduced through increased surveillance, chemoprevention, or prophylactic surgery (2). To assess knowledge of genetic testing for susceptibility to BOC, CDC has funded a review of BRCA1/2 testing in women with a family history of BOC (9) and an assessment of the clinical utility of BRCA1/2 counseling and testing by the U.S. Preventive Services Task Force (10). The findings in this report are subject to at least five limitations. First, information was not available for nonresponders. Whether responders differed from nonresponders with respect to demographics and other variables such as cancer family history is unknown. Second, because of the low consumer response rate (45%), a potential for bias might have been introduced; responders might have a different level of knowledge or interest in participating in testing or the survey. Third, the lag time between the campaign and the survey might not have been sufficient to allow those interested to pursue and complete testing. Fourth, quantifying the numbers of tests performed and appropriateness of testing was not possible because the information is proprietary and not available. Finally, objective data on the appropriateness of education, counseling, and tests ordered by providers are not available. In the United States, regulatory oversight of genetic testing is limited, and no process exists for review of the accuracy and impact of advertising claims about validity and utility of genetic tests. However, translation of genomic discoveries to medical practice continues to yield new applications for diagnosis, promise for new disease treatments and preventions, and increased consumer interest and demand for genetic testing. Public health agencies should provide information about genetic tests to educate consumers and providers and protect consumers by ensuring the safe and effective use of genetic tests. Collaboration among public health agencies, health-care providers, the clinical laboratory/biotechnology industry, and professional organizations will be required to develop a systematic approach for evidence-based assessment of the clinical validity and utility of genetic tests, identify gaps in knowledge, and to determine test efficacy, utilization, and access through postmarket surveillance. Such partnerships will be needed to support public health responses as genomics becomes more integrated into health promotion and disease prevention. Acknowledgments This report is based on contributions by J Mouchawar, MD, Kaiser Permanente, Denver, Colorado. B Bernhardt, MS, Johns Hopkins Medical Institutions, Baltimore, Maryland. D Irwin, PhD, Univ of North Carolina Center for Genomics, Chapel Hill, North Carolina. DJ Bowen, PhD, Fred Hutchinson Cancer Research Center; BB McGrath, PhD, Univ of Washington School of Nursing, Seattle; DL Doyle, MS, Genetics Svcs Section, Washington State Dept of Health. L Armstrong, PhD, Div of Cancer Prevention and Control, National Center for Chronic Disease Prevention and Health Promotion; K Peterson, MS, Office of Genomics and Disease Prevention, Office of the Director; A Faucett, MS, K Reed, MPH, Public Health Practice Program Office, CDC. References
Table 1 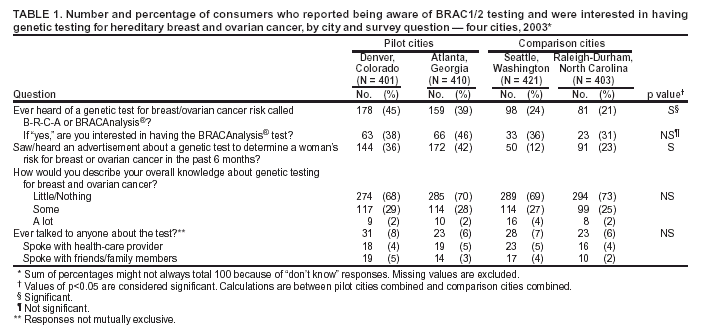 Return to top. Table 2 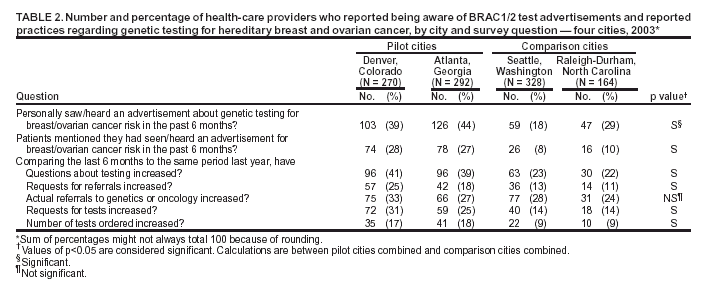 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 7/15/2004 |
|||||||||
This page last reviewed 7/15/2004
|