 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Progress Toward Elimination of Measles and Prevention of Congenital Rubella Infection --- European Region, 1990--2004The European Region (EUR) of the World Health Organization (WHO) comprises 52 member countries*, with an estimated population of 876 million. In 1998, the Regional Committee for EUR resolved to interrupt indigenous measles transmission by 2007 and reduce the incidence of congenital rubella syndrome (CRS) in all countries to <1 per 100,000 live births by 2010 (1). In 2002, progress toward these measles and rubella targets was further encouraged with development of the Strategic Plan for Measles and Congenital Rubella Infection in the WHO European Region, which outlines an integrated approach to achieving both disease targets by 2010 by implementing six key strategies (2). This report presents data on measles, rubella, and CRS control in EUR during 1990--2004 and summarizes progress halfway through the implementation of the strategic plan. Measles, Rubella, and CRS SurveillanceCountries in EUR submit measles, rubella, and CRS case counts annually to the WHO Regional Office for Europe by using the WHO/UNICEF joint reporting form§. Countries also have been encouraged to report clinically diagnosed measles cases monthly by age group, vaccination status, and laboratory confirmation and to report outbreaks. In EUR, clinically diagnosed rubella is a nationally notifiable disease in all countries except Austria, France, Germany, Monaco, and Turkey, which combined account for 25% of the EUR population. In 2003, regional surveillance guidelines for measles and congenital rubella infection were issued (3). At the national level, countries use different methods to collect measles and rubella data, including aggregate (i.e., reporting in broad age groups), case-based (i.e., individual case investigation), and sentinel physician reporting. In 2004, measles and rubella surveillance was based on aggregate monthly reported data on clinically diagnosed measles cases from 44 (85%) countries and case-based data from five (10%) countries; in addition, 51 (98%) countries provided annual case counts. The Computerized Information System for Infectious Diseases (CISID; available at http://data.euro.who.int/cisid) processes and presents this information. In 2004, five (10%) countries provided monthly measles surveillance reports on time (i.e., >80% of monthly reports received before the 25th of the following month), and 37 (71%) provided complete monthly surveillance reports (i.e., >80% of monthly reports received) (Table 1). In 2002, a regional laboratory network was created to provide laboratory support for measles, rubella, and CRS surveillance. Forty-seven (90%) countries are served by a National Measles/Rubella Laboratory, which is linked to one of three WHO European Regional Reference Laboratories appointed in 2003¶ or to the Global Specialized Laboratory (Table 1). Laboratory investigations are enhanced by using standardized diagnostic methods and reagents and by implementing a quality assessment program, including an annual accreditation review, proficiency testing, and monthly online reporting of laboratory indicators (with completeness of 70% for 2004). Measles and Rubella VaccinationEach year, countries provide information on routine coverage with the first dose of measles-containing vaccine (MCV1) among children aged 12--23 months and supplemental immunization activities (SIAs) for measles and rubella. In 2003, of 52 countries in EUR, 27 (52%) reported MCV1 coverage of >95% (Table 1), and 36 (69%) achieved MCV1 coverage of >90%. In 2004, all 52 countries had a routine 2-dose measles vaccination schedule, compared with 49 (96%) in 2001**. In 2004, a total of 47 (90%) countries used a rubella-containing vaccine; 45 (87%) used combined measles-mumps-rubella vaccine (MMR), one (2%) used measles-rubella vaccine (MR), and one (2%) used a single antigen rubella vaccine. In contrast, in 2001, a total of 39 (76%) countries used a rubella-containing vaccine. During 1990--2004, nine countries conducted SIAs; five countries used an MR vaccine for SIAs, three simultaneously offered rubella vaccination for women of childbearing age, and one used routine services to reach susceptible cohorts by using MMR (Table 2). Approximately 27.7 million persons were vaccinated during these SIAs. Measles, Rubella, and CRS IncidenceThe incidence of measles in EUR is cyclical, with a peak every 4 years; however, the incidence declined markedly from 36.2 per 100,000 population in 1990 to 3.2 in 2003 (Figure). During 1999--2004, a total of 17 measles outbreaks were reported, including outbreaks with >250 cases in Ireland during 2003--2004, Italy during 2002--2003, Switzerland in 2003, France in 2003, Germany in 2003 (4--8), and in some newly independent states (NIS). Although measles deaths are underreported, 10 deaths were reported both in 2002 and 2003 and seven in 2004. During 2002--2004, the proportion of persons with reported measles who were hospitalized ranged from 11% to 18%. In 2004, the provisional incidence of measles was 2.9 per 100,000 population, and 26 (50%) countries reported a measles incidence of <1 per 1,000,000 population (Table 1). The incidence of rubella remains high in EUR with short inter-epidemic periods. In 2003, a total of 304,320 rubella cases were reported; of these, 125,187 (41%) and 120,377 (40%) were reported from the Russian Federation and Romania, respectively. In 2001, 2002, and 2003, a total of 21, 14, and 12 CRS cases, respectively, were reported, totaling 47 cases; 15 (32%) were from the Russian Federation, and 17 (36%) were from Romania (9). Reported by: FX Hanon, DPharm, JS Spika, MD, MN Mulders, PhD, G Lipskaya, PhD, N Emiroglu, MD, World Health Organization, Regional Office for Europe, Copenhagen, Denmark. A Uzicanin, MD, P Strebel, MD, Global Immunization Div, National Immunization Program, CDC. Editorial Note:Substantial progress has been made in EUR toward better control of measles and rubella, but further efforts are needed to interrupt indigenous measles by 2010 and to reduce CRS incidence in all countries to <1 per 100,000 live births. The decline in reported measles incidence during 1990--2004 has occurred despite enhancements in surveillance and is the result of improvements in routine measles vaccination (e.g., introduction of routine 2-dose schedules throughout the region) and SIAs to reduce susceptibility among older children, adolescents, and young adults. In EUR, routine vaccination has been an integral part of public health services and a key prevention component of primary health care, but the level of measles and rubella control varies greatly. Finland was the first country to introduce a routine 2-dose MMR childhood vaccination program and eliminate measles, mumps, and rubella by sustaining high coverage since the early 1980s; a similar level of disease control appears to have been achieved in other Scandinavian and some central European countries using the same approach (10). However, certain countries in western Europe still have inadequate measles vaccination coverage to interrupt indigenous transmission; recent measles outbreaks have alerted health authorities to the impact this disease can have on children's health. Countries in central and eastern Europe and in NIS have undergone substantial economic adjustments, which have led to changes in health-care services, including reduced financial support for immunization services, resulting in difficulties in improving disease surveillance, sustaining high vaccination coverage, and introducing additional vaccines, including MMR vaccine. Large rubella outbreaks continue to occur in countries that only recently introduced rubella vaccination (e.g., Russian Federation and Romania). In many countries, CRS surveillance is not fully implemented, resulting in underestimates of CRS disease burden, both at country and regional levels. High routine 2-dose vaccination coverage (>95% in each subsequent birth cohort in all districts) with measles-containing vaccine is the key strategy to achieving and sustaining a high population immunity and eventually interrupting indigenous measles transmission in EUR; widespread use of combined vaccines (MR and MMR) throughout the region provides an opportunity to simultaneously achieve rubella elimination and reduce CRS incidence. SIAs have also been used in EUR to rapidly achieve high population immunity to measles and rubella by targeting age groups epidemiologically defined as having large numbers of susceptible persons. Countries in EUR use SIAs as one-time opportunities to strengthen routine vaccination services by providing staff training and improving program infrastructure and national program management capacity, including cold chain, vaccination delivery, injection safety, waste management, and surveillance. The further strengthening of disease surveillance will be essential to identifying disease burden and gaps in routine vaccination and to monitoring progress toward elimination targets. Although considerable progress has been made in ensuring access to quality laboratory services throughout EUR, further efforts are needed to improve timeliness and completeness of monthly measles surveillance reports. Regional efforts to improve surveillance have included emphasis on case-based monthly reporting of measles and rubella, enhancement of laboratory capacity, and provision of regular feedback to countries through newsletters, regional or subregional meetings, and CISID, with monthly updated information for the general public. In addition, the Vaccine Safety Net promotes access to websites with information about immunization at http://www.euro.who.int/vaccine/related/20040826_1. Acknowledgments This report is based, in part, on contributions by immunization program staff in all EUR member countries. References
* Andorra, Albania, Armenia, Austria, Azerbaijan, Belarus, Belgium, Bosnia and Herzegovina, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Georgia, Germany, Greece, Hungary, Iceland, Ireland, Israel, Italy, Kazakhstan, Kyrgyzstan, Latvia, Lithuania, Luxembourg, Malta, Monaco, Netherlands, Norway, Poland, Portugal, Republic of Moldova, Romania, Russian Federation, San Marino, Serbia and Montenegro, Slovakia, Slovenia, Spain, Sweden, Switzerland, Tajikistan, The Former Yugoslav Republic of Macedonia, Turkey, Turkmenistan, Ukraine, United Kingdom, and Uzbekistan. These are 1) achieve and sustain high coverage with 2 doses of measles vaccine through routine vaccination services; 2) provide a second opportunity for measles vaccination through supplementary immunization activities (SIAs) to populations susceptible to measles; 3) use the opportunity provided by measles SIAs to target populations susceptible to rubella with combined measles- and rubella-containing vaccine; 4) ensure protection to women of childbearing age by achieving high coverage with rubella vaccine; 5) strengthen measles, rubella, and CRS surveillance by timely case investigation and laboratory confirmation; and 6) improve the availability of high-quality information for health-care professionals and the public regarding the benefits and risks associated with vaccination. § Data from France are routinely obtained from Bulletin Épidémiologique Hebdomadaire (available at http://www.invs.sante.fr/beh). ¶ The regional reference laboratories are located at the National Public Health Laboratory, Luxembourg; the Robert Koch Institute, Berlin, Germany; and the G.N. Gabrichevsky Institute of Epidemiology and Microbiology, Moscow, Russian Federation. Health Protection Agency in London, U.K., serves as one of two global specialized reference laboratories (the other is at CDC in Atlanta, Georgia). ** In 2001, EUR comprised 51 countries. Armenia, Azerbaijan, Belarus, Georgia, Kazakhstan, Kyrgyzstan, Republic of Moldova, Russian Federation, Tajikistan, Turkmenistan, Ukraine, and Uzbekistan. Table 1 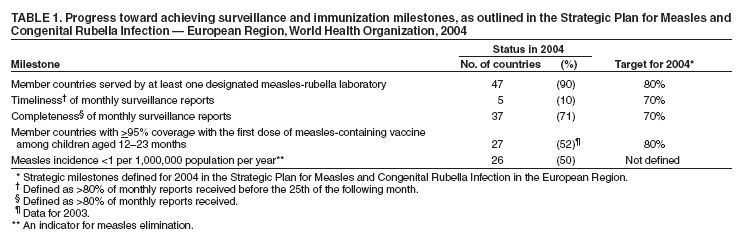 Return to top. Table 2 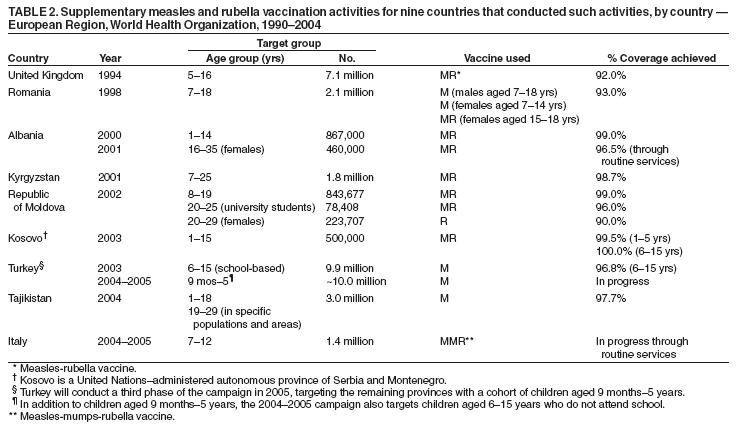 Return to top. Figure 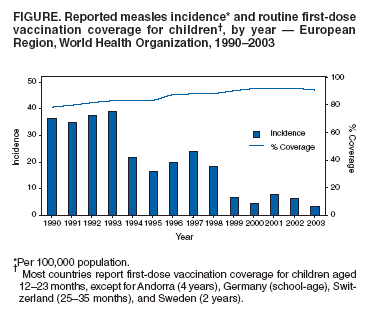 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 2/24/2005 |
|||||||||
This page last reviewed 2/24/2005
|