 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Lead Poisoning Associated with Use of Litargirio --- Rhode Island, 2003Lead can damage the neurologic, hematologic, and renal systems (1). Deteriorated leaded paint in older housing remains the most common source of lead exposure for children in the United States; however, other lead sources increasingly are recognized, particularly among certain racial/ethnic populations (2). In 2003, the Rhode Island Department of Health (RIDOH) recognized litargirio (also known as litharge or lead monoxide), a yellow or peach-colored powder used as an antiperspirant/deodorant and a folk remedy in the Hispanic community, as a potential source of lead exposure for Hispanic children. This report summarizes a case investigation of elevated blood lead levels (BLLs >10 µg/dL) associated with litargirio use among two siblings in Rhode Island, the public health action taken, and a survey of parents/guardians in three pediatric clinics in Providence, Rhode Island, to assess litargirio use. Findings underscore the importance of follow-up of elevated BLLs and thorough investigation to identify all lead sources. Case ReportIn May 2003, RIDOH and the Health & Education Leadership for Providence (HELP) Lead Safe Center investigated unexplained increases in BLLs in twin Hispanic boys aged 7 years (twins A and B). Annual BLL screenings for the twins since age 9 months were not elevated until June 2001, when twins A and B had elevated BLLs of 14 µg/dL and 15 µg/dL, respectively. Twin A's BLL increased to 42 µg/dL in May 2003, despite completed remediation of interior lead paint hazards in their home in June 2002 and of exterior lead hazards in May 2003, and provision of parental education about lead poisoning. Similarly, twin B's BLL increased to 26 µg/dL during the same period. In contrast, their younger brother's initial elevated BLL of 17 µg/dL in August 2001, at age 9 months, decreased to 8 µg/dL by November 2002. In May 2003, RIDOH and HELP Lead Safe Center staff conducted a home inspection, which detected litargirio in a small glass jar in the bedroom of the twins, who used the substance as an antiperspirant/deodorant. The youngest brother did not use litargirio and had a separate bedroom. After the litargirio tested positive for lead by a sodium rhodizonate field test, all litargirio was removed from the home, and a sample was sent to the state laboratory for confirmatory lead testing. The litargirio sample contained 790,000 parts per million (ppm) (79%) lead. Follow-up BLLs decreased for twin A (27 µg/dL in June, 22 µg/dL in August, and 13 µg/dL in November) and twin B (22 µg/dL in June, 17 µg/dL in August, and 9 µg/dL in November). The twins' visiting grandmother from the Dominican Republic had introduced litargirio into their home and also had given it to the family of their two female cousins, aged 1 and 5 years. In June 2002, the older girl had a BLL of 24 µg/dL, and the younger girl had a BLL of 32 µg/dL. Previous annual BLL screenings for the older girl were not elevated. In July 2002, after a home inspection revealed lead paint hazards, their parents implemented lead hazard control measures. However, the girls BLLs increased to 29 µg/dL and 44 µg/dL, respectively, by January 2003. The older sister used litargirio sporadically until the family ran out of the product in January 2003, after which her BLLs decreased to 20 µg/dL in March, 15 µg/dL in April, and 7 µg/dL in November. Although the younger girl had not used litargirio, she shared a bedroom with her older sister and likely ingested litargirio residue on various surfaces through hand-to-mouth activity. Her BLLs also decreased to 33 µg/dL in March, 29 µg/dL in April, and 16 µg/dL in November after her sister discontinued using litargirio. Public Health ActionLitargirio is available locally in botanicas (i.e., shops selling herbs) and bodegas (i.e., grocery stores) located in Hispanic communities. It is manufactured and/or packaged by laboratories in the Dominican Republic and sold in small, clear, plastic packets labeled "litargirio" (Figure). A litargirio sample purchased by RIDOH staff from a local botanica contained 360,000 ppm (36%) lead. RIDOH issued a statewide health alert on June 30, 2003, warning the public to stop using litargirio and advising pregnant and nursing women and children who used this product to obtain a BLL test. The media provided coverage in both English and Spanish. RIDOH notified CDC and the Food and Drug Administration (FDA) about the litargirio cases and, on October 2, FDA issued a warning to consumers about litargirio. RIDOH notified the Dominican Republic Secretary of Public Health about the high levels of lead in litargirio imported from the Dominican Republic. SurveyTo assess litargirio use in the Hispanic community in Providence, RIDOH and CDC conducted a convenience survey of parents/guardians in three hospital-based pediatric clinics over a 2-week period (weekdays) during January--February 2004. Hospital A (a pediatric clinic and pediatric dental clinic) was surveyed during January 5--9 and 12--16. Hospital B (a pediatric clinic) was surveyed during February 9--13 and 17--20. All parents/guardians were approached to determine whether they were eligible for the survey (i.e., considered themselves Hispanic, were a parent/guardian, lived with a child, and were aged >18 years). A screening questionnaire was administered to 1,025 persons; 599 (58%) were deemed eligible. Of those eligible, 584 (98%) participated in the survey. Among participants, 157 (27%) had heard about litargirio; of those, 134 (85%) were Dominicans. Among the 134 Dominican participants who had heard about litargirio, the majority (104 [78%]) heard about it as a tradition from their country of origin. Of the 40 participants with a personal or family history of litargirio use, 38 (95%) were Dominicans who typically used the substance while growing up in the Dominican Republic. No Dominican participants reported current or recent personal use of litargirio. Furthermore, no study participant reported using litargirio before or after the health alert. No additional cases of litargirio-associated lead poisoning have been reported to RIDOH or CDC. Reported by: D Silva, Health & Education Leadership for Providence (HELP) Lead Safe Center; J Tourangeau, St Joseph's Hospital Lead Clinic & HELP Lead Safe Center, Providence; R Aglione, M Angeloni, MBA, C Brackett, W Dundulis, MS, Rhode Island Dept of Health. Div of Emergency and Environmental Health Svcs, National Center for Environmental Health; N Reyes, MD, EIS Officer, CDC. Editorial Note:Litargirio is used in the manufacture of batteries, glass, and ceramics; in the vulcanizing of rubber; and as a paint pigment (3--5). Dominicans, particularly those from rural areas, use it as an antiperspirant/deodorant and as a traditional remedy for burns and fungal infections of the feet. This report, the first to describe lead poisoning associated with use of litargirio, demonstrates how a thorough investigation of elevated BLLs led to the discovery of litargirio, a previously unreported source of lead exposure. Although deteriorated leaded paint in older housing remains the main source of childhood lead exposures, other sources should be considered, particularly when a child's elevated BLL does not respond to remediation of residential lead paint hazards. As described in this report, the BLLs of the twins' youngest brother decreased after residential lead paint hazards were remediated, but the twins' BLLs continued to increase, suggesting exposure to a different lead source. BLL elevations during or immediately after remediation or abatement are uncommon in Rhode Island because of strict control of the process. Certain racial/ethnic populations at risk for lead exposure through use of traditional or folk remedies (6--9) might fail to disclose use of these products when asked about use of "traditional or folk remedies," rather than by product name. In this report, the twins' mother repeatedly denied use of "traditional or folk remedies" because she considered litargirio an ordinary product (i.e., deodorant), not a remedy. RIDOH now inquires specifically about use of litargirio when visiting Hispanic families of children with elevated BLLs. Data regarding dermal absorption of inorganic lead compounds in humans is limited but reportedly substantially lower than absorption through inhalation or ingestion (1). Although litargirio was applied to the skin of these children, most of the product probably was ingested through hand-to-mouth behavior after contact with the product or with contaminated surfaces. Twin A, who had the higher BLL, sucked his thumb, supporting this premise. The findings from the convenience survey are subject to at least two limitations. First, the survey sampled only persons seeking pediatric care at the three pediatric clinics; therefore, the results might not be generalizable to all Hispanic communities in Rhode Island. Second, health warnings about the use of litargirio might have biased participant responses and underestimated the prevalence of litargirio use. However, to minimize participant bias, Hispanic interviewers conducted the survey and collected no identifiers. The survey results suggest that the prevalence of litargirio use in Rhode Island was minimal. Later attempts by RIDOH staff to purchase litargirio from botanicas or bodegas failed to locate any litargirio. Because of these findings, RIDOH took no further action. Conversely, in New York City (NYC), the NYC Department of Health and Mental Hygiene was able to purchase litargirio from five of eight botanicas visited in NYC after learning about the Rhode Island litargirio cases. One of the five litargirio samples tested contained lead (430,000 ppm [43%] lead). A public warning was issued, and botanica owners were required to remove all litargirio from their stores. References
Figure 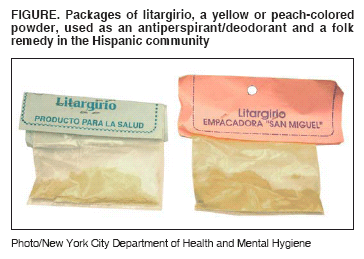 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 3/10/2005 |
|||||||||
This page last reviewed 3/10/2005
|