 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Anhydrous Ammonia Thefts and Releases Associated with Illicit Methamphetamine Production --- 16 States, January 2000--June 2004Anhydrous ammonia, a colorless gas with a pungent, suffocating fumes, is used primarily as an agricultural fertilizer and industrial refrigerant (1). Anhydrous ammonia is also a key ingredient for illicit methamphetamine (meth) production in makeshift laboratories. Exposure to anhydrous ammonia can be immediately dangerous to life or health (1,2). Anhydrous ammonia generally is not available for sale to the public; states require a license for purchase. Because of this, many illicit meth producers (i.e., "cookers") resort to stealing anhydrous ammonia. If released into the environment, anhydrous ammonia can cause acute injuries to emergency responders, the public, and the cookers themselves. In addition, when handled improperly, anhydrous ammonia can be explosive and deadly. This report describes examples of anhydrous ammonia thefts associated with illicit meth production, summarizes ammonia theft events reported to the Agency for Toxic Substances and Disease Registry (ATSDR), and suggests injury prevention recommendations, such as installing valve locks or fencing on unattended tanks and donning appropriate personal protective equipment (PPE) when responding to releases. ATSDR maintains the Hazardous Substances Emergency Events Surveillance (HSEES) system to collect and analyze data about the public health consequences (i.e., morbidity, mortality, and evacuations) of hazardous substance--release events*. The information in this report is based on events reported to HSEES from 16 state health departments during January 1, 2000--June 30, 2004§. Case ReportsWashington. In April 2004, at approximately 5:50 a.m., nearly 1,500 pounds of anhydrous ammonia were released during an attempted theft at a cold-storage facility. The release occurred as perpetrators broke off the valve of a 6,100-gallon tank. The suspected perpetrator, who sustained chemical burns on his torso, was taken to an emergency department. A responding firefighter sustained respiratory irritation because of a breach in his Level A hazardous materials (HazMat)¶ suit. Several roads were closed, businesses were evacuated, and a train was delayed while company employees, a HazMat team, and local police and fire departments responded. Approximately 12 persons were evacuated for 8 hours, and nearby residents were told to shelter in place. Eight uninjured responders were decontaminated on the scene after the event. Missouri. In October 2003, at approximately 7:45 p.m., anhydrous ammonia was released during an attempted theft at an agricultural facility. A firefighter and a police officer responding to the release both experienced respiratory irritation. The police officer was not wearing PPE at the time of injury; the firefighter became symptomatic before donning his firefighter turn-out gear** with respiratory protection. The police officer was transported to a hospital for treatment but not admitted; the firefighter was administered oxygen on the scene. The fire department declared the scene safe for reentry 3 hours after the event. Alabama. In February 2002, at approximately 3:00 a.m., nearly 150 gallons of anhydrous ammonia were released during an attempted theft at a food-processing plant. The perpetrator tried unsuccessfully to siphon the ammonia into an oxygen cylinder. No victims or injuries were reported. The local fire department responded and declared the scene safe for reentry 4 hours after the event. Summary of Surveillance DataOf the 40,349 events reported to the HSEES system during January 1, 2000--June 30, 2004, a total of 1,791 (4%) were associated with illicit meth production. Of the 1,791 meth events, at least 164 (9%) were known to have been caused by anhydrous ammonia theft with the intention of meth production (6). These ammonia theft events were reported in 10 of the 16 HSEES states, with Iowa (64 [39%]) and Missouri (57 [35%]) reporting the most events. The most common locations of ammonia theft events were commercial (88 [52%]) and agricultural areas (51 [31%]). Nearly half (74 [45%]) of these events occurred during May--August. Sundays had the highest frequency of events (31 [19%]). Of the 157 (96%) events for which time of occurrence was known, more events occurred during midnight through 5:59 a.m. (59 [38%]) than during any other time. Of the 164 ammonia theft events, 36 (22%) resulted in a total of 85 injured persons. Persons most frequently injured were members of the general public (38 [45%]) and police officers (27 [32%]). The 85 persons injured (victims) had 110 reported injuries, most frequently respiratory irritation (68 [62%]) and eye irritation (19 [17%]). Most (48 [56%]) victims were treated at a hospital but not admitted, and 18 (21%) were treated on the scene. No deaths occurred. A total of 27 (16%) of the 164 ammonia theft events involved ordered evacuations, of which 17 had a known number of evacuees. A total of 2,146 persons were known to have evacuated, ranging from two to 300 persons per event (median: 20 persons). The median duration of these evacuations was 2.8 hours (range: <1--8 hours). Decontamination of potentially exposed persons was necessary in 13 events. A total of 57 persons underwent decontamination; 48 (84%) were emergency responders, and nine (16%) were employees (e.g., farmers or agricultural workers). Reported by: T Arant, Alabama Dept of Public Health. C Henry, Missouri Dept of Health and Senior Svcs. W Clifford, Washington Dept of Health. DK Horton, MSPH, S Rossiter, MPH, Div of Health Studies, Agency for Toxic Substances and Disease Registry. Editorial Note:Meth, a powerfully addictive stimulant, is produced in illicit, makeshift laboratories (7). Anhydrous ammonia is a key ingredient used in illicit meth production. Although most anhydrous ammonia is used for legitimate purposes, a small percentage is diverted to meth manufacturing. Those involved in illicit production of meth often resort to stealing anhydrous ammonia from areas where it is stored and used (e.g., farms, industrial refrigeration systems, and railroad tanker cars) (8). These thefts often lead to releases when valves are left open as ammonia is being siphoned; ammonia is transferred inappropriately into makeshift containers, such as propane tanks used on barbeque grills; plugs are removed from ammonia lines at refrigeration facilities; or the wrong hoses or fittings are attached to storage containers (8). As liquid anhydrous ammonia is released into ambient air, it expands substantially, forming large vapor clouds that behave as a dense gas. This dense gas can travel along the ground instead of immediately rising into the air and dispersing, thereby increasing the potential for exposure to humans (8). Symptoms of anhydrous ammonia exposure include eye, nose, and throat irritation; dyspnea; wheezing; chest pain; pulmonary edema; pink frothy sputum; skin burns; vesiculation; and frostbite. Exposure can be fatal at high concentrations (2). Farmers and merchants often are unaware of an anhydrous ammonia theft unless a large-scale release occurs (9). Nearly half of these HSEES events occurred during agricultural season. In addition, 38% occurred during early morning hours, and 19% occurred on Sundays, when commercial establishments usually are closed. Furthermore, the amount of anhydrous ammonia stolen in each event was small compared with the total volume of the tank. The findings in this report are subject to at least two limitations. First, reporting of any event to HSEES is not mandatory; therefore, participating state health departments might not be informed about every event. In addition, because meth laboratories are illicit, sources (primarily law enforcement officials) might hesitate to report events that could jeopardize criminal investigations. Second, HSEES is not conducted in all states; therefore, HSEES data might not represent populations in other areas. Several additives are being developed and used to help curb anhydrous ammonia thefts and releases. For example, researchers are studying an additive that could be mixed into the ammonia, rendering it useless for meth production (Iowa State University, unpublished data, 2005). In addition, Glotell (Royster Clark, Inc.; Norfolk, Virginia), a new, commercially available additive is being used as a marking agent, leak detector, and theft deterrent; this additive causes objects that contact the released anhydrous ammonia to turn fluorescent pink, thus helping farmers to easily detect which tanks have been subject to ammonia leaks or thefts. In addition, this additive reportedly turns meth pink and decreases its potency, causing the meth cooker more difficulty in selling the final product. Several additional measures can help farms and industries deter anhydrous ammonia theft and prevent accidental releases (8) (Box). Emergency responders to an anhydrous ammonia release should select the proper PPE before entering a release zone. Positive-pressure, self-contained breathing apparatus is recommended in response situations that involve exposure to potentially unsafe levels of ammonia (1). In addition, chemical-protective clothing is recommended because ammonia can cause skin irritation and burns (1). Acknowledgments The findings in this report are based, in part, on contributions by C Kelley, Colorado Dept of Health. D Cooper, Iowa Dept of Public Health. D Dugas, MPH, Louisiana Dept of Health and Hospitals. N Rice, Minnesota Dept of Health. R Mozingo, Mississippi State Dept of Health. J Savrin, New Jersey Dept of Health and Senior Svcs. R Wilburn, MPH, New York State Dept of Health. S Giles, North Carolina Dept of Health and Human Svcs. T Tsongas, PhD, Oregon Public Health Svcs. L Phillips, Rhode Island Dept of Health. R Harris, Texas Dept of Health. W Ball, PhD, Utah Dept of Health. J Drew, Wisconsin Dept of Health and Family Svcs. References
* An HSEES event is the release or threatened release of a hazardous substance into the environment in an amount that requires (or would have required) removal, cleanup, or neutralization according to federal, state, or local law (3). A hazardous substance is one that can reasonably be expected to cause an adverse health effect. Alabama, Colorado, Iowa, Louisiana, Minnesota, Mississippi, Missouri, New Jersey, New York, North Carolina, Oregon, Rhode Island, Texas, Utah, Washington, and Wisconsin. § An earlier HSEES analysis examined data for 1996--1999 because 1996 was the first year several meth events began appearing in the system (4). Data as of June 30, 2004, were the most recent data available when the analysis was conducted; data for 2004 are still considered preliminary. ¶ Includes self-contained breathing apparatus plus totally encapsulating chemical-resistant clothing (i.e., permeation resistant) (5). ** Includes a helmet and face piece, coat, pants, boots, gloves, hood, and a self-contained breathing apparatus (5). Box 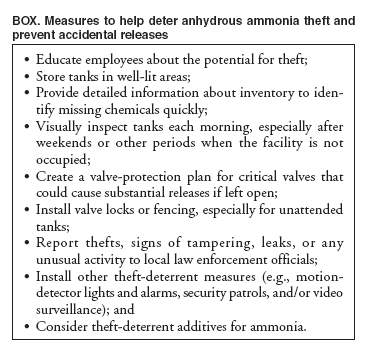 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 4/14/2005 |
|||||||||
|