 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Surveillance for Laboratory-Confirmed, Influenza-Associated Hospitalizations --- Colorado, 2004--05 Influenza SeasonThe number of annual hospitalizations for influenza and pneumonia associated with influenza viruses in the United States is estimated at 95,000 (1); however, no state-based or national surveillance system exists to monitor these events in all age groups, and population-based numbers of laboratory-confirmed, influenza hospitalizations are unknown. Certain existing surveillance systems provide population-based national estimates of influenza-related hospitalizations based on sampling methodology (i.e., the National Hospital Discharge Survey) or sentinel surveillance; however, these systems are not timely, population-based for all ages, and available at the state level. The Emerging Infections Program (EIP) conducts population-based surveillance for laboratory-confirmed, influenza-related hospitalizations of persons aged <18 years in 11 metropolitan areas, and the New Vaccine Surveillance Network (NVSN) provides population-based estimates of laboratory-confirmed influenza hospitalization rates among children aged <5 years who were prospectively enrolled and tested for influenza in three sentinel counties. The U.S. Department of Health and Human Services recommends that states develop strategies to monitor influenza-related hospitalizations (2). This report describes a surveillance system for laboratory-confirmed, influenza-associated hospitalizations in all age groups in Colorado that was implemented for the 2004--05 influenza season. The findings indicate that implementation of statewide, population-based surveillance for influenza-associated hospitalizations is feasible and useful for assessing the age-specific burden of serious influenza-associated morbidity and the relative severity of influenza seasons. On September 30, 2004, influenza-ssociated hospitalizations became a condition reportable by Colorado health-care providers. An influenza-associated hospitalization was defined for surveillance purposes as a hospital admission accompanied by an appropriate laboratory test result for influenza, including results from rapid diagnostic tests. Population estimates for 2003 (overall 4.6 million) by age group were obtained from the Colorado Department of Local Affairs and used to compute annual age-specific rates of influenza-associated hospitalization. Case reports of influenza-associated hospitalization contained the same core variables that are collected for all reportable diseases in Colorado, including patient identifying, locating, and demographic information; name of reporting agency; physician name and contact information; specimen collection date, specimen type, and test type; test result and date, and report date, Reporting of notifiable diseases by 68 hospitals in Colorado is performed primarily by infection-control practitioners (ICPs). Many ICPs enter data directly into the state's web-based disease reporting system; however, others fax reports to the Colorado Department of Public Health and Environment (CDPHE) or report directly to local health departments. During the 2004--05 influenza season, ICPs ascertained cases of influenza-associated hospitalization by reviewing clinical laboratory and admission information routinely available to them. ICPs entered 74% of reported influenza-associated hospitalizations directly into the state's reporting system; state or local health department staff members entered the remaining 26%. Since the 1999--00 influenza season in Colorado, influenza surveillance data have been compiled weekly from multiple sources (e.g., influenza-like illness [ILI] reported by sentinel providers and one health maintenance organization; outbreaks of influenza in nursing homes; absenteeism reported by sentinel schools; and influenza virus typing and subtyping data from state and clinical laboratories) and disseminated via an electronic summary to local health departments. However, none of these influenza surveillance methods are population-based, and none focus on hospitalization. As of April 16, 2005, a total of 964 influenza-associated hospitalizations had been reported by 50 hospitals, producing a rate of 21.0 per 100,000 persons during the 2004--05 influenza season. Reported cases peaked during the week ending February 19, 2005 (Figure), which was also the peak week for the percentage of patient visits for ILI reported by sentinel health-care providers in Colorado (CDPHE, unpublished data, 2005). Influenza virus type--specific testing results were available for 896 (92.9%) reported cases, of which 86.3% were influenza A and 13.7% were influenza B. The most frequently reported test type was rapid influenza testing (88.0%), followed by direct fluorescent antibody (5.8%) and viral culture (5.6%). The highest influenza-associated hospitalization rates were in persons aged >80 years (207.3 per 100,000 population) and children aged <6 months (183.0 per 100,000), followed by persons aged 70--79 years (78.0 per 100,000) and children aged 6--23 months (66.3 per 100,000) (Table). Persons aged >60 years accounted for 51.4% of reported cases. The median time from specimen collection to disease report was 2 days, with 86% of cases reported within 7 days. Reported by: K Gershman, MD, Colorado Dept of Public Health and Environment. Editorial Note:Previous efforts to determine the impact of influenza on hospitalizations were based on statistical modeling methods (e.g., using national hospital discharge survey data) (1,3--6). The overall rate of influenza-associated hospitalizations (21.0 per 100,000 population) reported in Colorado during the 2004--05 influenza season through the new statewide notifiable disease surveillance is similar to published estimates based on national hospital discharge data. These estimates include a mean of 36.8 per 100,000 population (range: 7.8--71.4) for primary listed pneumonia and influenza hospitalizations for influenza seasons 1979--80 through 2000--01 (1) and a mean of 49 per 100,000 population (range: 8--102) for excess pneumonia and influenza hospitalizations for influenza seasons 1969--70 through 1994--95 (3). Estimates based on hospital discharge data are not available nationally for at least 12 months and on the state level for several months; however, statewide surveillance for influenza-associated hospitalizations in Colorado provided real-time, population-based incidence of influenza-associated hospitalization. Surveillance also confirmed the high risk for hospitalization among the youngest and oldest populations. The findings in this report are subject to at least four limitations. First, influenza testing is not likely to be performed on all persons hospitalized with acute respiratory illness or with exacerbations of chronic respiratory or cardiovascular disease resulting from influenza infection. Therefore, surveillance for hospitalizations based on positive influenza testing underestimates the number of influenza-associated hospitalizations. Second, the sensitivity of rapid influenza tests is lower than that of viral culture and varies by test (7), which also contributes to underestimates of influenza-related illness. Third, rapid influenza tests can have low positive predictive value both early and late in the influenza season, when the prevalence of circulating influenza viruses is low (7). Finally, the data in this report are from one influenza season; the incidence of influenza-associated hospitalization and possibly the resources needed to conduct surveillance will vary depending on the severity of the influenza season. CDC maintains and coordinates a national influenza surveillance system that allows public health officials to know when and where influenza activity is occurring, determine what types of influenza viruses are circulating, detect changes in the influenza viruses, track influenza-related illness, and measure the impact of influenza on overall mortality in the United States (8). However, none of these national components provide population-based influenza-related hospitalization rates for all age groups. Surveillance for influenza-associated hospitalizations can provide multiple benefits to Colorado and other states that might adopt similar systems. The system provides improved ability to assess the severity of influenza seasons, track the time course of the season, determine which populations are most affected by severe influenza-related illness, and focus prevention and control efforts on those populations. A national surveillance system similar to the one implemented in Colorado could provide data to 1) monitor and describe the incidence, distribution, and basic epidemiologic characteristics of hospitalizations related to influenza virus infection; 2) guide future influenza immunization policy (e.g., expansion of immunization recommendations for children); 3) rapidly recognize influenza seasons in which the number of hospitalizations appears unusually high; and 4) help identify an influenza pandemic and direct public health response. The recent development and widespread use of rapid influenza testing makes it feasible and desirable to use case reporting based on positive laboratory testing to monitor influenza-associated hospitalizations. Acknowledgments The findings in this report are based, in part, on contributions by M Evdemon-Hogan, MSPH, B Stone, MSPH, Colorado Dept of Public Health and Environment. A Postema, MPH, L Brammer, MPH, T Uyeki, MD, Influenza Br, National Center for Infectious Diseases, CDC. References
Table 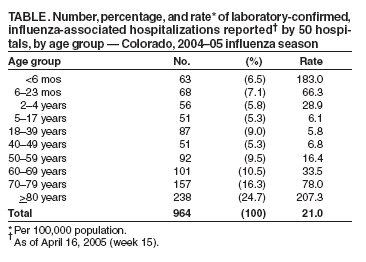 Return to top. Figure 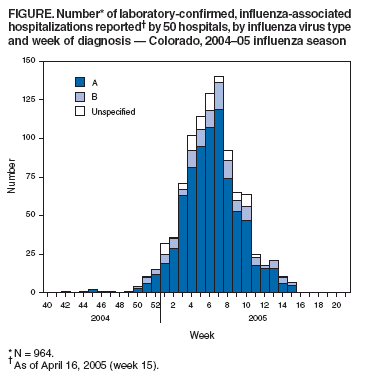 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 6/2/2005 |
|||||||||
|