 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Progress Toward Poliomyelitis Eradication --- Afghanistan and Pakistan, 2008Afghanistan and Pakistan, two of the four remaining countries where wild poliovirus (WPV) transmission has never been interrupted (1),* represent one epidemiologic reservoir. During 2008, both countries continued to conduct coordinated supplemental immunization activities (SIAs) against type 1 WPV (WPV1) and type 3 WPV (WPV3) using oral polio vaccine (OPV). Much of Afghanistan remained polio-free in 2008, with the exception of the conflict-affected South Region. In Pakistan, however, WPV transmission increased, particularly after WPV1 reintroduction into polio-free areas of Punjab Province. In total, 149 WPV cases (31 in Afghanistan and 118 in Pakistan) were confirmed in 2008, compared with 49 cases in 2007. Serious security problems in areas along the common border limited access by vaccination teams to large numbers of children in the two countries. In Pakistan, continued managerial and operational problems impeded full implementation of SIAs and adversely affected vaccination coverage in areas not affected by security problems. This report updates previous reports (1,2) and describes polio eradication activities in Afghanistan and Pakistan during January--December 2008. Further progress toward interruption of WPV transmission in Afghanistan and Pakistan will require continued measures to overcome access problems in conflict-affected areas of both countries and improvements in the quality of SIAs and delivery of routine immunization services in Pakistan. Immunization ActivitiesIn 2007, the most recent year for which data were available, routine immunization coverage of infants with 3 doses of trivalent oral poliovirus vaccine (OPV3) by age 12 months was 83% overall in both Afghanistan and Pakistan (3). However, acute flaccid paralysis (AFP) surveillance data§ suggest that actual routine OPV3 coverage was much lower nationally and varied widely by political area (province, territory, or region) in both countries. Based on AFP surveillance data reported during 2008, routine OPV3 coverage among children aged 6--23 months with nonpolio AFP in Afghanistan was 69% in the Central Region, 66% in the East Region, 47% in the Southeast Region, and 13% in the South Region; in Pakistan, coverage was 72% in Punjab Province, 60% in Northwest Frontier Province (NWFP), 53% in Sindh Province, and 37% in Balochistan Province. Large-scale house-to-house SIAs targeting children aged <5 years and using trivalent and/or monovalent type 1 (mOPV1) and type 3 OPV, depending on the epidemiologic situation, continued in Afghanistan and Pakistan during 2008 (Table 1). Afghanistan conducted four national immunization days (NIDs) and five subnational immunization days (SNIDs) in the East, Southeast, and South regions along the border with Pakistan, covering about 50% of the national population of children aged <5 years. Pakistan conducted five NIDs and six SNIDs in the main WPV transmission areas, targeting 40%--50% of the total population aged <5 years. To improve SIA monitoring and coverage evaluation, finger marking (with an indelible ink pen) of children vaccinated during SIAs was introduced in both countries in 2008. Comparison of finger-marking rates to post-campaign surveys based on caretaker recall showed persisting gaps in vaccination coverage, particularly in Pakistan (e.g., in Sindh Province, 72%--84% by finger marking versus >95% by caretaker recall). As a result of deteriorating regional security, the percentage of children aged <5 years living in inaccessible areas (considered too dangerous by the World Health Organization [WHO] and the local government to conduct an SIA) increased during 2008 in both countries. During January--November 2008, the percentage of children aged <5 years living in inaccessible areas in Pakistan increased from 11% to 13% in NWFP and from 21% to 38% in eight large districts and tribal agencies (Swat, Bajour, Mohmand, Charsadda, Peshawar, parts of Kohat, Kurram, and South Waziristan).¶ Security restrictions specifically prevented United Nations staff members who supervise and monitor SIAs from entering >90% of districts in the South Region and 50% of districts in the East and Southeast in Afghanistan, and 80% of districts in NWFP in Pakistan, a worsening of the situation since 2007. AFP SurveillanceIn 2008, AFP surveillance quality indicators exceeded WHO operational targets.** The annual nonpolio AFP rate (per 100,000 population aged <15 years) at the national level was 7.6 in Afghanistan (range among the eight regions: 5.0--11.4) and 6.5 in Pakistan (range among the five provinces/territories: 3.9--11.2), an increase from 2007, during which the nonpolio AFP rates were 6.9 and 5.6, respectively. The percentage of AFP cases with adequate stool specimen collection was 93% in Afghanistan (range by region: 85%--97%) and 90% in Pakistan (range by province/territory: 82%--94%) (Table 2). The polio laboratory at the National Institute of Health (NIH) in Islamabad, Pakistan, provides laboratory support for AFP surveillance in both countries, including genomic sequencing. During 2008, the NIH laboratory processed 3,465 stool samples from Afghanistan and 13,086 from Pakistan. WPV IncidenceIn Afghanistan, 31 polio cases were reported during 2008, compared with 17 cases in 2007 (Figure, Table 2). Among polio cases reported during 2008, 25 (81%) were caused by WPV1 and six (19%) by WPV3, compared with six (35%) and 11 (65%), respectively, during 2007. Of the 31 polio cases reported in 2008, 27 (87%) were among children aged <36 months; seven (23%) had received no OPV doses, nine (29%) had received 1--3 of any OPV doses, and 15 (48%) had received >4 of any OPV doses. In Pakistan, the reported number of polio cases increased from 32 in 2007 to 118 during 2008 (Figure, Table 2). In 2008, 81 (69%) cases were caused by WPV1 and 37 (31%) by WPV3, compared with 19 (59%) and 13 (41%), respectively, during 2007. During 2008, 102 (86%) of the 118 cases involved children aged <36 months; among those children, 10 (9%) had received no OPV doses, 16 (13%) had received 1--3 of any OPV doses, and 92 (78%) had received >4 of any OPV doses. Genetic sequencing data from 2008 indicate the persistence of endemic WPV circulation in two main transmission zones of both countries, and a recurrence of WPV transmission in previously polio-free areas of Punjab Province, Pakistan. The northern transmission zone includes most of NWFP and the Federally Administered Tribal Areas (FATA) in Pakistan and bordering areas in eastern Afghanistan (Figure). In 2008, 56 cases were reported from this zone, including an outbreak of 33 WPV3 cases, centered in Peshawar, the provincial capital of NWFP, during the second half of the year. The WPV3 outbreak followed a series of SIAs using mOPV1 during 2007--2008 targeting WPV1 transmission in central NWFP. The southern transmission zone forms a corridor from the West and South regions of Afghanistan into Pakistan through Balochistan and southern Punjab into Sindh (including Karachi). In 2008, a total of 58 cases were reported from this zone. In addition, a WPV1 outbreak involving 31 cases occurred in northern Punjab during July--November 2008 after nearly 2 years without a reported WPV1 case. Only one outbreak-related case was reported from southern Punjab. The outbreak was linked genetically to two separate WPV1 clusters, one circulating in NWFP and the other in Sindh Province. Before the outbreak, only five NIDs had been conducted in 2007 in northern Punjab compared with five NIDs and five SNIDs in southern Punjab. In addition, routine immunization coverage and SIA management and implementation in northern Punjab had declined during 2007. Reported by: World Health Organization (WHO) Eastern Mediterranean Regional Office Egypt, Cairo; WHO Afghanistan, Kabul; WHO Pakistan, Islamabad; Polio Eradication Dept, WHO, Geneva, Switzerland. Global Immunization Div, Div of Viral Diseases, National Center for Immunization and Respiratory Diseases, CDC. Editorial Note:During 2008, despite continued intensive polio eradication activities in Afghanistan and Pakistan, WPV1 and WPV3 continued to circulate in the two shared transmission zones of both countries. In addition, WPV1 was reintroduced into previously polio-free areas of northern Punjab Province, Pakistan. However, most of Afghanistan continues to be free of endemic WPV transmission. Similarly, after efforts to improve strategy implementation in Sindh Province, Pakistan, no WPV1 cases have been reported there since August 2008. Two critical factors hamper efforts to interrupt WPV transmission in both countries: conflicts affecting increasingly large parts of the border area between the countries, and operational and management issues impeding the quality of SIAs in Pakistan. In the northern transmission zone, large areas of NWFP and FATA in Pakistan and the East region of Afghanistan often were too dangerous to conduct SIAs. Access in the South Region of Afghanistan decreased further during 2008, after some improvements in late 2007 (2). In Sindh and Balochistan provinces, which did not have serious security problems, political and managerial issues adversely affected supervision and accountability, resulting in failure to fully and properly implement SIAs and continued WPV transmission. Maintaining high levels of immunity in areas where WPV transmission has been interrupted also remains a priority, to prevent recurrence of outbreaks such as the one in Punjab. In addition to continued support from the international polio eradication partnership, interruption of WPV transmission in Afghanistan and Pakistan will require overcoming one of the most important remaining challenges in polio eradication globally: the barriers to access and vaccination of children in large, remote, and security-compromised areas. Efforts to engage political and tribal leaders will need to be enhanced to secure access and safe passage of vaccination teams to these areas. In the interim, critical improvements are needed in the quality of SIAs and delivery of routine immunization in both countries. References
* The other two countries where WPV transmission has never been interrupted are India and Nigeria. Mass campaigns conducted for a brief period (days to weeks) in which 1 dose of oral poliovirus vaccine is administered to all children aged <5 years, regardless of vaccination history. Campaigns can be conducted nationally or in portions of the country. § Vaccination histories of children aged 6--23 months with AFP who do not test as WPV positive are used to estimate OPV coverage of the overall target population. These AFP data are used to verify national reported routine immunization coverage estimates. ¶ Total populations aged <5 years in the districts were 5.9 million in NWFP and 1.95 million in the eight districts and tribal agencies. ** The quality of AFP surveillance is monitored by three performance indicators: 1) detection rate of AFP cases not caused by WPV, 2) the proportion of AFP cases with adequate stool specimens, and 3) the proportion of stool specimens processed in a WHO-accredited laboratory. Current WHO operational targets for countries with endemic polio transmission are a nonpolio AFP detection rate of at least two cases per 100,000 population aged <15 years and adequate stool-specimen collection from >80% of AFP cases, in which two specimens are collected at least 24 hours apart, both within 14 days of paralysis onset, and shipped on ice or frozen ice packs to a WHO-accredited laboratory, arriving in good condition.
Table 1 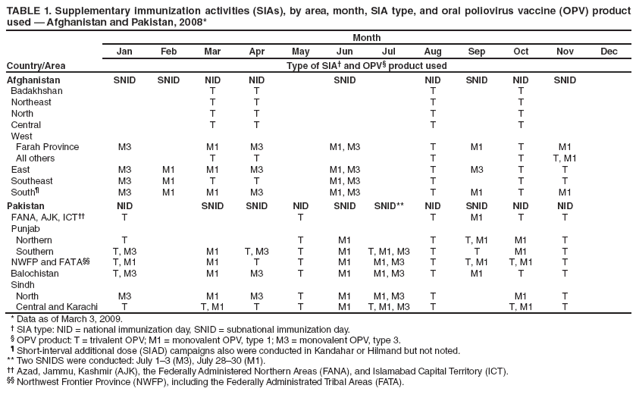 Return to top. Table 2  Return to top. Figure 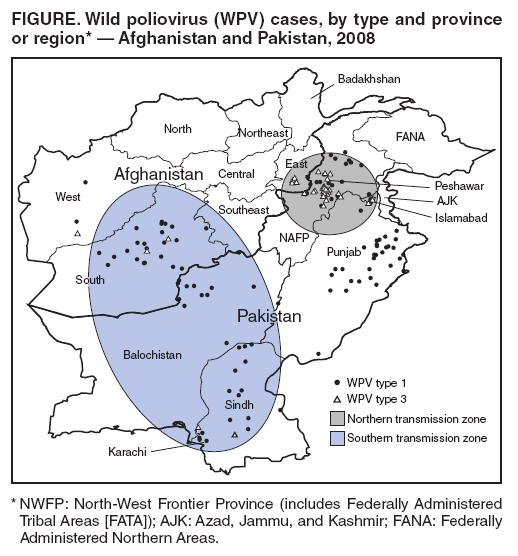 Return to top.
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 3/5/2009 |
|||||||||
|