Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Laboratory-Acquired Vaccinia Virus Infection --- Virginia, 2008
Vaccinia virus (VACV) is the live viral component of smallpox vaccine. Inadvertent exposure to VACV can result in infection, and severe complications can occur in persons with underlying risk factors (e.g., pregnancy, immunodeficiencies, or dermatologic conditions) (1). The Advisory Committee on Immunization Practices (ACIP) recommends smallpox vaccination for laboratory workers who handle nonhighly attenuated VACV strains or other orthopoxviruses (e.g., monkeypox, cowpox, or variola) (2). On July 8, 2008, CDC was notified by a Virginia physician of a suspected case of inadvertent autoinoculation and VACV infection in an unvaccinated laboratory worker. This report describes the subsequent investigations conducted by the Virginia Department of Health and CDC to identify the source of infection and any cases of contact transmission. Of the patient's 102 possible contacts, seven had underlying risk factors for developing serious vaccinia infection. Investigators found no evidence of contact transmission and, based on the results of molecular typing, further concluded that the patient had been exposed to a VACV strain that had contaminated the seed stock from the laboratory where the patient worked. This case underscores the importance of adherence to ACIP vaccination recommendations for laboratory workers and use of safety precautions when working with nonhighly attenuated VACV (3).
Case Report
On July 5, 2008, a man in his twenties who worked in a laboratory at an academic institution in Virginia went to a local urgent care clinic. He reported swelling of cervical lymph nodes and pain and inflammation of his right earlobe associated with purulent discharge beginning July 2, followed on July 3 by a feverish feeling and swelling of his left eye with no change in his vision. The patient was prescribed cephalexin for presumed bacterial infection and prednisone for swelling. However, on July 6, his symptoms worsened, and he went to a hospital emergency department. The patient was given bacitracin for his eye and discharged. That night, he noted pustular lesions at similar stages of development on his right ear and left eye (Figure), and also on his chest, shoulder, left arm, and right leg.
On July 7, the patient returned to the emergency department with increasing eye pain and mild photophobia and received a diagnosis of right auricular/pinnal cellulitis and suspected periorbital cellulitis. Prednisone was discontinued, and he was admitted to the hospital for treatment with intravenous vancomycin, ceftriaxone, and pain medications. The same day, an ophthalmology consultation was obtained for left-sided severe preseptal cellulitis, confirmed by computed tomography scan. Biopsy of the conjunctival lesion revealed acute necrotizing conjunctivitis. Slit lamp examination revealed no apparent corneal abrasions and a clear anterior chamber in the left eye, with slight loss of visual acuity. Because the patient's eye infection appeared consistent with keratitis, ceftriaxone was discontinued, vancomycin was continued, and the patient was started on piperacillin/tazobactam and clindamycin.
On July 8, an infectious disease physician who was consulted raised the possibility of suspected VACV infection, among other more common viral or bacterial etiologies, because of histopathologic changes noted in the patient's eye specimens. The consulting physician elicited from the patient that he worked in a cancer research laboratory that handled mice infected with VACV. The physician contacted CDC, which contacted the Virginia Department of Health. Upon further investigation, the patient was determined to have worked with VACV during June 26--28, 4--6 days before symptom onset. This information was inconsistent with the patient's statement during his initial interview on admission the previous day, when he said he recalled last working with VACV in mid-May. Specimens from the patient's eye, ear, arm, and chest were sent to the Virginia Laboratory Response Network. The patient met the CDC surveillance case definition for ocular vaccinia (1).
On July 9, a computed tomography scan revealed worsening of the left preseptal infectious process with intraorbital involvement. On July 10, pending receipt of viral testing, 800 mg acyclovir was administered to the patient intravenously. After receipt of diagnostic testing results, vaccinia immune globulin was not administered because the patient was improving. The patient went on to make a full recovery and returned to his laboratory work in August 2008.
Laboratory Analysis
On July 9, the Virginia Laboratory Response Network tested lesion scrapings from the patient using real-time polymerase chain reaction and detected the presence of nonvariola orthopoxvirus DNA signatures. CDC subsequently confirmed the VACV infection. However, molecular typing of VACV from the patient specimens, performed at CDC, indicated that the patient was infected with a strain (VACV Western Reserve strain) that differed from the VACV strain reportedly used in the laboratory's experiments (the recombinant construct OVA-vac). Because the patient and laboratory VACV strains did not match, investigators had to consider the possibility that the patient might have acquired his VACV infection from another source, most likely within the institution's laboratory complex.
Additional VACV specimens were collected both from the laboratory in which the patient worked and from other laboratories in the academic institution's research complex, and an investigation was launched to identify the source of exposure. CDC analyzed samples of all the virus stocks used at the academic institution and detected a contaminant virus in the OVA-vac stock from the laboratory in which the patient worked that closely resembled the VACV strain isolated from the patient.
Occupational Health Investigation
During August 4--5, investigators interviewed three persons separately regarding experiments performed at the laboratory during June and July: the patient, the laboratory director, and a student who worked with the patient during June 26--28, when the patient's exposure to VACV was thought to have occurred. Although the academic institution's occupational health clinic annually provided education on workplace safety and offered smallpox vaccination to all laboratory workers who handled nonhighly attenuated VACV strains or other orthopoxviruses, neither the patient nor the student had plans to be vaccinated. The laboratory director was not up-to-date with his VACV vaccination (last vaccinated >10 years previously).
Representatives of the occupational health and biosafety team at the academic institution were interviewed to review their biosafety, VACV-use, and vaccination policies for laboratory personnel. Investigators found that safety protocols were in place. However, as a result of this incident, changes in laboratory procedures regarding VACV were made. Before the incident, the academic institution offered VACV counseling and vaccination only to personnel who specifically requested vaccination, even if the employee's written work profile indicated VACV use. As a result of the incident, the academic institution now offers counseling and education to all personnel with occupational exposure to VACV. Vaccination is then offered to laboratory workers without medical contraindications, and a declination form is completed for laboratory workers who decline the vaccine. In addition, changes have been made to the academic institution's laboratories to better reflect CDC biosafety recommendations (4).
Contact Investigation
Recognizing that inadvertent transmission of VACV can occur through contact with lesion exudates, investigators interviewed the patient to identify his potential close contacts from July 2, when symptoms began, through the period he was hospitalized. A close contact was defined as any person with direct physical contact with the patient or his linens, trash, or clinical specimens. Initially, 102 persons with possible exposure to the patient's lesions were identified: eight personal contacts, 12 laboratory workers, and 82 hospital workers.
Fifty-five (54%) of the 102 possible contacts were identified as potentially having contact with the patient's lesion exudates and were interviewed by the Virginia Department of Health or members of the institution's infection control staff regarding symptoms of possible VACV infection (e.g., fever, malaise, myalgia, and lymphadenopathy) and risk factors for severe infection. These 55 close contacts included eight personal contacts, 12 laboratory workers, and 35 hospital workers. All were asked to report any symptoms or illnesses for 14 days after their exposure. Seven of the 55 (four personal contacts and three hospital workers) had risk factors for severe infection (i.e., pregnancy, immunodeficiencies, or dermatologic conditions). However, no secondary VACV infections were detected.
Reported by: E Davies, MPH, L Peake, MD, D Woolard, PhD, C Novak, MD, Virginia Dept of Health; K Hall, MD, RT Leonard, PhD, R Allen, PhD, Virginia. M Reynolds, PhD, W Davidson, MPH, C Hughes, MPH, V Olson, PhD, S Smith, MS, H Zhao, MD, Y Li, PhD, K Karem, PhD, I Damon, MD, PhD, Div of Viral and Rickettsial Diseases, National Center for Zoonotic, Vector-Borne, and Enteric Diseases; A MacNeil, PhD, A Roess, PhD, EIS officers, CDC.
Editorial Note:
In 1972, routine childhood vaccination against smallpox was halted because of a declining probability of smallpox importation, reduced likelihood of spread following importation, and occasional untoward side effects of vaccination (5). In 2003, members of the military, selected health-care workers, public health personnel, and first responders began receiving smallpox vaccinations as part of bioterrorism preparedness (6). From 1972 to 2003, laboratory workers were the only group recommended for periodic smallpox vaccination in the United States. ACIP currently recommends smallpox vaccination at least every 10 years for laboratory workers who handle cultures or animals infected with nonhighly attenuated VACV or other orthopoxviruses (e.g., monkeypox, cowpox, or variola)* (7).
Laboratory-acquired VACV infections are not nationally notifiable conditions but often are reported to CDC when virus confirmation is required for diagnosis. These laboratory-acquired infections typically occur in unvaccinated workers (2). During 2005--2007, five cases of laboratory-acquired VACV infection were reported to CDC (1). No known contact transmission of VACV was reported from these laboratory-acquired infections; however, instances of contact transmission of VACV from smallpox vaccinees to close contacts, including children and intimate partners, has occurred (8). Adherence to ACIP recommendations by laboratorians often is dependent on interpretations of the risks for VACV laboratory exposure by laboratory directors (who might not be fully aware of the pathogenic properties of VACV in humans), concerns over adverse events associated with vaccination, and the extent of VACV education provided to laboratory workers (2). After the incident described in this report, VACV laboratory procedures were changed, and counseling and education was extended to all laboratory workers with occupational exposure to VACV.
Laboratory-acquired exposure to VACV can be associated with a high inoculum and can occur through a route (e.g., ocular) with a high risk for complications (9). In the event of an exposure, the affected body part should be washed immediately; eyewash protocols should be followed for ocular exposure. The laboratory worker should then report the incident to the laboratory director or to the occupational health clinic. Depending on the timing and circumstances of the exposure and status of the inoculated site, administration of postexposure vaccination, vaccinia immune globulin, or antivirals might be indicated to attenuate adverse clinical outcomes associated with VACV infection (7).
Clinicians should maintain a high index of suspicion for VACV infection when evaluating vesiculopapular rashes in patients who are laboratory workers handling nonhighly attenuated VACV strains or are their close contacts. Suspected cases of VACV infection should be reported to state or local health departments for diagnostic guidance. Further characterization of viruses can be performed at specialized reference laboratories such as the poxvirus laboratory at CDC (telephone: 404-639-4129). Contact VACV transmission is uncommon (5.9 cases per 100,000 vaccinations) (3,6,10), and infection control measures are effective in preventing such transmission (7); therefore, contact investigations should be limited to persons who might have had contact with lesion exudates, whether or not they have risk factors for severe VACV infection.
References
- CDC. Surveillance guidelines for smallpox vaccine (vaccinia) adverse reactions. MMWR 2006;55(No. RR-1).
- CDC. Laboratory-acquired vaccinia exposures and infections---United States, 2005--2007. MMWR 2008;57:401--4.
- Sepkowitz KA. How contagious is vaccinia? N Eng J Med 2003;348:439--46.
- US Department of Health and Human Services, CDC, National Institutes of Health. Biosafety in microbiological and biomedical laboratories. 5th ed. Washington, DC: US Department of Health and Human Services, CDC, National Institutes of Health; 2007. Available at http://www.cdc.gov/od/ohs/biosfty/bmbl5/bmbl5toc.htm.
- CDC. Supplement: collected recommendations of the Public Health Service Advisory Committee on Immunization Practices. MMWR 1972;21.
- CDC. Secondary and tertiary transfer of vaccinia virus among U.S. military personnel---United States and worldwide, 2002--2004. MMWR 2004;53:103--5.
- CDC. Recommendations for using smallpox vaccine in a pre-event vaccination program: supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR 2003;52(No. RR-7).
- CDC. Household transmission of vaccinia virus from contact with a military smallpox vaccinee---Illinois and Indiana, 2007. MMWR 2007;56:478--81.
- Lewis FM, Chernak E, Goldman E, et al. Ocular vaccinia infection in laboratory worker, Philadelphia, 2004. Emerg Infect Dis 2006;12:134--7.
- Neff JM, Lane JM, Fulginiti VA, Henderson DA. Contact vaccinia---transmission of vaccinia from smallpox vaccination. JAMA 2002;288:1901--5.
* Smallpox vaccination is no longer recommended for laboratory workers handling highly attenuated poxvirus strains because these strains either are unable to replicate or replicate poorly in mammalian host cells and, therefore, do not create productive infections in healthy persons.
FIGURE. Left eye and right ear of a man with laboratory-acquired vaccinia virus infection --- Virginia, 2008
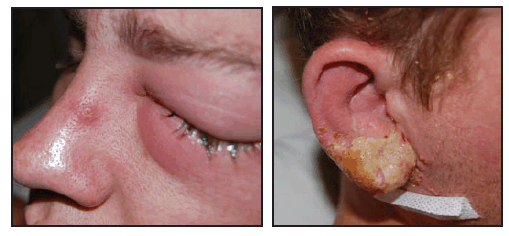
Photos/Virginia Department of Health
Alternative Text: The figure above shows the left eye and right ear of a man with laboratory acquired vaccinia virus infection from Virginia in 2008. The man worked for a laboratory at an academic institution in Virginia. He went to a local urgent care clinic reporting swelling of cervical lymph nodes and pain and inflammation of his right earlobe associated with purulent discharge beginning July 2, followed on July 3 by a feverish feeling and swelling of his left eye with no change in his vision. The patient was prescribed cephalexin for presumed bacterial infection and prednisone for swelling However, on July 6, his symptoms worsened, and he went to a hospital emergency department. The patient was given bacitracin for his eye and discharged. That night, he noted pustular lesions at similar stages of development on his right ear and left eye.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services. |
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 7/30/2009


