Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Performance of Rapid Influenza Diagnostic Tests During Two School Outbreaks of 2009 Pandemic Influenza A (H1N1) Virus Infection --- Connecticut, 2009
During May 2009, a few weeks after 2009 pandemic influenza A (H1N1) infection was first detected in the United States (1), outbreaks among students from two schools were detected in Greenwich, Connecticut. Staff members from Greenwich Hospital and the Connecticut Department of Public Health collected data on symptoms for 63 patients and submitted nasopharyngeal washings for testing using a rapid influenza diagnostic test (RIDT) for influenza A and B and real-time reverse transcription--polymerase chain reaction (rRT-PCR) assay, thereby affording an opportunity to assess the field performance of the RIDT. A total of 49 patients had infections with pandemic influenza A (H1N1) confirmed by rRT-PCR. This report summarizes the findings from this performance assessment, which indicated that, compared with rRT-PCR, the sensitivity of the RIDT for detecting infection in patients with 2009 pandemic influenza A (H1N1) was 47%, and the specificity was 86%. Sensitivity and specificity did not vary substantially by the presence or absence of CDC-defined influenza-like illness (ILI) or by time from symptom onset to specimen acquisition. In this group of patients, although positive RIDT results performed well in predicting confirmed infection with pandemic H1N1 virus (positive predictive value: 92%), negative tests did not accurately predict the absence of infection (negative predictive value: 32%). These results affirm recent CDC recommendations against using negative RIDT results for management of patients with possible 2009 pandemic influenza A (H1N1) infection (2).
During April 29--May 1, 2009, 78 students from a private school (school A) near Greenwich, Connecticut, participated in a class trip to Pennsylvania. Several students became sick with a respiratory illness. Because infection with 2009 pandemic influenza A (H1N1) was suspected, upon returning home, 11 of the students, a sibling, and two other students went to the Greenwich Hospital for outpatient influenza testing and treatment.
During May 18--20, 133 students and eight teachers from a public school (school B) in Greenwich traveled to a camp in Connecticut. Among these students, 36 visited the camp infirmary with fever, headache, or fatigue. The Greenwich Health Department asked physicians at the hospital to assist with testing the students for pandemic H1N1. A total of 67 students and staff from school B became ill, and 49 of these patients went to the hospital for influenza testing.
A total of 63 patients (14 students from school A and 49 students and staff from school B) were tested for influenza at the hospital. A standard symptom survey was completed by a physician for each patient after which a nasopharyngeal washing was performed by an experienced respiratory therapist trained in the procedure. All samples were placed in viral transport media and sent to the Connecticut Department of Public Health laboratory for influenza virus detection by rRT-PCR. Rapid screening for influenza A and B was performed concurrently at the hospital laboratory using the Remel Xpect Flu A&B test (Remel Products, Lenexa, Kansas) according to manufacturer's instructions (3). Although the number of ill persons who eventually received antiviral therapy is unknown, all nasopharyngeal washings were obtained before initiation of therapy.
Of the 63 patients tested by RIDT, 49 patients, 11 (79%) from school A and 38 (78%) from school B, were found to have 2009 pandemic influenza A (H1N1) infection by rRT-PCR (Figure). Of the 49 patients with confirmed infection, 23 (47%) tested positive (eight from school A and 15 from school B) and 26 (53%) tested negative for 2009 pandemic influenza A (H1N1) by RIDT. Among 11 patients with positive rRT-PCR tests from school A and 38 from school B, the numbers of positive RIDT tests were 8 (73%) and 15 (39%) respectively.
Among the 14 patient samples from both schools that tested negative by rRT-PCR, three were from students at school A, and 11 were from school B. Of the 14 rRT-PCR negative specimens, two tested positive by RIDT (one from school A and one from school B). The overall sensitivity of the RIDT was 23 of 49 (47%), and the specificity was 12 of 14 (86%). The positive predictive value was 23 of 25 (92%), and the negative predictive value was 12 of 38 (32%).
The schools did not differ significantly with respect to percentage of patients with confirmed pandemic H1N1 by rRT-PCR, severity of symptoms, interval between the onset of symptoms and collection of specimens for testing, or overall RIDT positivity rate. Among all the patients tested by RIDT, no significant differences between true positives and false negatives were seen with respect to ILI.* In RIDT positive and RIDT negative patients with pandemic H1N1, the median interval from symptom onset to specimen collection was 36 hours. Of the 34 patients with washings obtained ≤36 hours from the onset of symptoms, 16 (47%) were RIDT positive; of the 15 patients with washings collected after 36 hours of symptoms, seven (47%) were positive. RIDT test performance was assessed for patients with and without CDC-defined ILI (Table). The sensitivity and specificity were approximately the same for the two groups (48% versus 44% and 88% versus 83%, respectively).
Reported by: JR Sabetta, MD, J Smardin, L Burns, MPH, K Barry, MS, Greenwich Hospital Section of Infectious Diseases; C Baisley, MPH, T Mahoney, MS, D Travers, MSN, Greenwich Dept of Health; T Brennan, J Fontana, PhD, Connecticut Dept of Public Health Laboratory; T Rabatsky-Ehr, MPH, ML Cartter, MD, Connecticut Dept of Public Health.
Editorial Note:
When cases of 2009 pandemic influenza A (H1N1) began appearing in the United States in April 2009, several RIDTs had been in common use in the United States as point-of-care tests for seasonal influenza, but the performance of these tests in patients infected with 2009 pandemic influenza A (H1N1) virus was unknown. CDC has since reported varying sensitivities of RIDTs in retrospective analyses of rRT-PCR positive respiratory samples, from 40%--69%. In these analyses, RIDT sensitivity was positively associated with the titer of virus in the sample (4).
The analysis in this report of pandemic H1N1 cases at two schools determined that the RIDT used detected less than half the cases confirmed by rRT-PCR. The low sensitivity and low negative predictive value of the test during these outbreaks highlight the limitations of using this test alone to establish diagnosis and aid clinical management. These results affirm current recommendations not to use negative RIDT results to rule out pandemic H1N1 or to make infection control decisions (2).
Rapid tests differ in their sensitivity and specificity for detecting seasonal influenza in respiratory specimens but generally have low to moderate sensitivity compared with viral culture or rRT-PCR. Previous RIDT studies have described the performance of the QuickVue Influenza A+B test (Quidel Corporation, San Diego, California) for detecting seasonal influenza in three different populations during 2008. Sensitivity when compared with rRT-PCR was low for all populations (median: 27%; range: 19%--32%) (5).
The RIDT used in the current study has a reported sensitivity of 92.5% and a specificity of 100% for the diagnosis of seasonal influenza A by nasopharyngeal wash (3). This investigation yielded much lower sensitivity (47%) and specificity (86%) in patients having confirmed infection with 2009 pandemic influenza A (H1N1) virus.
The findings in this report are comparable to recently reported observations of low performance of RIDTs in patients with pandemic H1N1. In a report of hospitalized patients in California, rapid antigen test results were positive in 67% of cases of pandemic H1N1 tested (6). In an assessment of rapid testing compared with rRT-PCR conducted on 6,090 patient samples from the New York City area, the sensitivity and specificity for the detection of 2009 pandemic influenza A (H1N1) virus by rapid antigen testing, using the BinaxNOW Influenza A&B test (Binax, Inc., Scarborough, Maine) and the 3M Rapid Detection Flu A+B test (3M, St. Paul, Minnesota) were 17.8% and 93.6% respectively (7). A recent report from the Naval Health Research Center described screening 3,066 clinical samples from service personal with influenza-like illness; of those screened, 767 rapid test results by QuickVue Influenza A+B test were available for comparison with rRT-PCR results (8). Of 39 patients with pandemic H1N1, 20 were RIDT positive, with a 51% sensitivity; for seasonal influenza A the sensitivity was 63% for H1N1 and 31% for H3N2. Specificity was 99% for all three subtypes when compared with rRT-PCR.
The results of these studies and the findings in this report affirm that a negative result for this rapid test does not rule out 2009 pandemic influenza A (H1N1) virus infection in an individual with symptoms consistent with influenza. Factors that might decrease the performance of rapid influenza antigen tests include improper specimen collection, not testing the recommended clinical sample (e.g., nasal versus nasopharyngeal swab), quality of the specimen, prolonged time from illness onset to specimen collection (because viral shedding decreases over time), and improper handling and storage of the specimen before testing. The reason for the suboptimal detection of 2009 pandemic influenza A (H1N1) by the RIDT used in this study was not specifically determined but did not appear to be related to differences in the interval (median: 36 hours for both groups) from onset of symptoms to specimen collection or to the severity of symptoms. As with all screening tests, the positive and negative predictive values of RIDTs are dependent on the prevalence of the disease in the population.
The findings in this report are subject to at least one limitation. The assessment involved a limited number of patients from two small outbreaks. The results should be viewed in this context. In other field situations (e.g., with other disease prevalences, collection and transport methods, or using other RIDTs), RIDTs might have different performance characteristics.
RIDTs can be an important tool for patient care during the normal influenza season because they usually provide results within 30 minutes. In addition, these tests can be used to make decisions about isolating or cohorting patients in health-care settings and recommending or restricting patient movements in outpatient settings. They might be especially important for hospitals limited by the expense of rRT-PCR and in identifying influenza during outbreaks in defined patient groups, such as those in schools or nursing homes. However, if used for management of patients with possible pandemic H1N1 virus infection, false-negative test reports might result in inappropriate exposure of susceptible persons to infected patients. Additional large studies to better characterize the performance of RIDTs for detection of infection in patients with pandemic H1N1 virus and improvements in rapid testing for pandemic H1N1 are needed.
Acknowledgment
The findings in this report are based, in part, on data provided by the Westchester County Dept of Health, New Rochelle, New York.
References
- CDC. Swine influenza A (H1N1) infection in two children---southern California, March--April 2009. MMWR 2009;58:400--2.
- CDC. Interim guidance for the detection of novel influenza a virus using rapid influenza diagnostic tests. Atlanta, GA: US Department of Health and Human Services, CDC, 2009. Available at: http://www.cdc.gov/h1n1flu/guidance/rapid_testing.htm.
- Remel, Inc. Remel Xpect Flu A&B package insert. Lenexa, KS: Remel, Inc.; 2008.
- CDC. Evaluation of rapid influenza diagnostic tests for detection of novel influenza A (H1N1) virus---United States, 2009. MMWR 2009;58:826--9.
- Uyeki TM, Presad R, Vutotich C, et al. Low sensitivity of rapid diagnostic tests for influenza. Clin Infect Dis 2009;48(9):e89--e92.
- CDC. Hospitalized patients with novel influenza A (H1N1) virus infection---California, April--May, 2009. MMWR 2009;58:536--41.
- Ginocchio CC, Zhang F, Manji R, et al. Evaluation of multiple test methods for the detection of the novel 2009 influenza A (H1N1) during the New York City outbreak. J Clin Virol 2009;45:191--5.
- Faix DJ, Sherman SS, Waterman SH. Rapid-test sensitivity for novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med 2009;361:728--9.
* CDC ILI surveillance case definition: fever (≥100ºF [≥37.8ºC]), plus cough, sore throat, or bothin the absence of another known cause of illness.
FIGURE. Number of confirmed* cases of 2009 pandemic influenza A (H1N1) virus infections after school trips, by school, date of hospital visit, and result of rapid influenza diagnostic test† --- Connecticut, May 2009
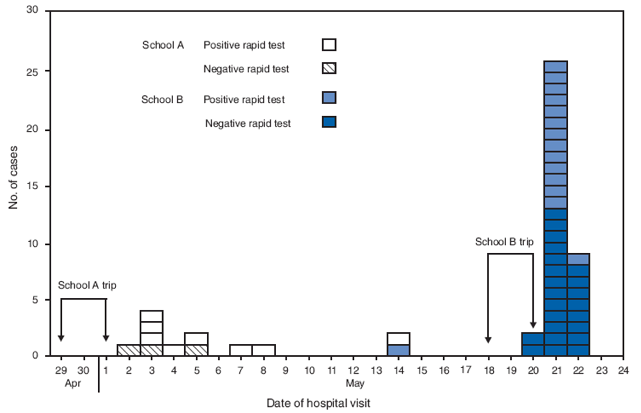
* By real-time reverse transcription--polymerase chain reaction assay; all patients tested negative for seasonal influenza.
† Remel Xpect Flu A&B test (Remel Products, Lenexa, Kansas).
Alternative Text: The figure above shows the number of confirmed cases of 2009 pandemic influenza A (H1N1) virus infections following school trips, by school, date of hospital visit, and result of rapid influenza diagnostic test in Connecticut, May 2009. Of the 63 patients tested overall, 49 patients, 11 (79%) from school A and 38 (78%) from school B, were found to have 2009 pandemic influenza A (H1N1) infection confirmed by real-time reverse transcription-polymerase chain reaction.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services. |
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 9/24/2009


