Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Fatal Poisoning Among Young Children from Diethylene Glycol-Contaminated Acetaminophen --- Nigeria, 2008--2009
On November 18, 2008, the Nigerian Federal Ministry of Health (FMOH) received a report of 13 cases of unexplained acute renal failure among children from a hospital in Lagos state. Several of the patients had been exposed to a liquid acetaminophen-based teething medication. On November 21, officials from the Nigerian National Agency for Food and Drug Administration and Control (NAFDAC) discovered diethylene glycol (DEG) in four batches of the teething medication manufactured during August--October 2009. DEG is a toxic alcohol used in brake fluid, paint, and household cleaning products, and has been used illegally as a cheap substitute solvent in drug manufacturing. Previous DEG poisonings resulting from contamination of medications have been reported in the United States, Nigeria (1990), Panama, and other countries (1--3), and acute renal failure (ARF) is a known manifestation of DEG poisoning. An investigation was launched by the Nigeria Field Epidemiology and Laboratory Training Program (N-FELTP), CDC, and the Food and Drug Administration (FDA). This report summarizes the results of the investigation, which identified 57 cases of DEG poisoning among children aged ≤3 years during August 2008--January 2009, of whom 54 died. Of the 57 children with DEG poisoning, 96% had exposure to the acetaminophen-based teething medication (My Pikin). DEG contamination was identified in six bottles of the medication from patient households and four batches from the facility in which the medication was manufactured. Well-developed and strictly enforced pharmaceutical quality control measures and training programs can prevent DEG-associated large-scale poisoning events (4,5).
The initial 13 cases of ARF reported to FMOH occurred over a period of 2 weeks, and represented a large increase over the baseline incidence of ARF at the hospital of 1--2 cases per month. All the cases had occurred in children aged ≤3 years. Hospitals in Kaduna and Osun states reported similar clusters of ARF. Because several of the ill patients had been exposed to the acetaminophen-based teething medication before disease onset, the medication became the focus of the initial investigation. On November 21, after NAFDAC officials discovered DEG contamination in batches of the medication, a full product recall was initiated, and the manufacturing facility was shut down. FMOH requested assistance from CDC for the epidemiologic investigation, and NAFDAC asked FDA to inspect the facility that had manufactured the teething medication. CDC and FDA investigators arrived in mid-January, after the product recall had been issued, and after the outbreak had peaked (Figure).
To ascertain cases and determine the scope of the poisoning, N-FELTP and FMOH conducted active, hospital-based surveillance in the three states (Kaduna, Lagos, and Osun) to identify physician-diagnosed ARF cases of any etiology in children aged <18 years. No additional cases were detected from FMOH nationwide passive surveillance. By January 8, 2009, 111 physician-diagnosed ARF cases of any etiology had been identified, and four additional cases were identified by field investigators through hospital-based surveillance in the three states, for a total of 115 ARF cases.
To differentiate background cases of ARF (of any etiology) from ARF cases associated with DEG poisoning, investigators focused further investigations on ARF cases that were unexplained. A confirmed case of unexplained ARF was defined as acute-onset anuria or oliguria of unknown etiology lasting ≥24 hours, with onset after August 1, 2008 (the manufacturing date of the first known DEG-contaminated batch). Cases were classified solely on the clinical observation of urine output, and no laboratory confirmation of ARF was available. N-FELTP or CDC investigators used a standard questionnaire to interview 71 parents, guardians, or physicians of the 115 ARF patients; the remaining 44 families could not be contacted or located. Information collected included illness characteristics, underlying health conditions, medical evaluation, and medication exposures. During interviews, residual medications in households were collected and sent to FDA's Forensic Chemistry Center for analysis by gas chromatography--mass spectrometry for DEG.
Based on 71 completed interviews, 57 (80%) patients met the confirmed case definition for unexplained ARF. Of these, 37 (65%) patients were male, and 56 (98%) were previously healthy (one patient had sickle cell disease). Median patient age was 12 months (range: 1 week--27 months). Of the 57 patients, 55 (96%) had exposure to the teething medication, and 16 (28%) had received the medication after the product recall in Nigeria was announced. A total of 54 patients (95%) died.
Of 46 (81%) patients with available information, the median time from exposure to ARF onset was 5.6 days (range: 0--24 days).* For 52 of the patients with information available, the mean interval between ARF onset and death was 6.8 days (range: 1--19 days). No biologic samples from patients could be obtained because of the high fatality rate and retrospective nature of the investigation. Among the 57 patients, 24 (42%) underwent dialysis and two (4%) received fomepizole, an antidote for ethylene glycol toxicity. No particular treatment combination appeared to improve survival.
During the interviews, 34 medication bottles from 13 different patients were collected, including seven bottles of the teething medication. DEG contamination (17%--21% DEG by weight)† was identified in six of those bottles. Laboratory analyses identified a second contaminated medication (0.5% DEG) in another acetaminophen-based syrup by a different manufacturer. One patient had exposure to both medications. The remaining 26 medications tested negative for DEG contamination. Although the exact mechanism of contamination was not identified, facility inspection revealed multiple errors common to previous DEG-associated large-scale poisoning events (6), including 1) use of unknown or unapproved raw material suppliers for propylene glycol, 2) lack of certificates of analysis from suppliers to certify the ingredient's identity and purity, 3) failure to perform propylene glycol identity testing, 4) failure to analyze finished product for DEG, and 5) failure to track the distribution of finished product. The product recall resulted in the confiscation of 7,616 bottles of the teething medication, representing 51% of approximately 15,000 contaminated bottles produced during August--October 2008. Investigators convened key stakeholders within FMOH and from national and international agencies in February to produce additional press releases for radio, television, and print media to support the product recall. In addition, investigators recommended further investigation of the second brand of syrup.
Reported by: A Abubukar, MBBS, E Awosanya, DVM, O Badaru, PhD, S Haladu, DVM, P Nguku, MBChB, Nigerian Field Epidemiology and Laboratory Training Program. P Edwards, MPA, R Noe, MN, MPH, M Teran-Maciver, MSN, A Wolkin, MSPH, L Lewis, MD, National Center for Environmental Health; M Nguyen, EIS Officer, CDC.
Editorial Note:
This report describes Nigeria's second and largest DEG-associated large-scale poisoning since 1990. The hallmark of DEG poisoning is ARF. The temporal association between ARF and reported exposure to the implicated medication among 96% of the children in this event, combined with discovery of DEG contamination in samples of the implicated medication from patients' homes, indicate that the medication was the poisoning source. A substantial proportion of the children with DEG poisoning (28%) were given the implicated teething medication after the product recall was announced, even though the recall targeted pharmacies and consumers. Product recalls will never completely eliminate the risk for harmful exposure after a product is distributed widely. Safety measures must be directed primarily at preventing contamination during manufacture and before sale of the product.
During the past 70 years, at least 12 occurrences of DEG contamination in oral and topical medications have resulted in at least 450 deaths (1--3). These large-scale poisonings have occurred predominantly in developing countries and have been associated with inadequate adherence to safe manufacturing practices, lack of enforcement of safe practices, or what appear to be intentionally deceptive drug manufacturing practices (7). In all but one of the 12 DEG mass-poisoning events (7), propylene glycol or glycerin was the intended diluent. Because these diluents have very different manufacturing methods and neither produces DEG as a byproduct, simple errors of cross-contamination during manufacturing cannot account for the frequent substitution of DEG in pharmaceuticals. Economically motivated substitution was suspected in several prior outbreaks, because DEG is less expensive than pharmaceutical-grade solvents.
Use of safe manufacturing practices might have prevented this event. Simple, rapid, and low-cost assays using thin-layer chromatography (TLC) have been developed to detect and quantify DEG contamination (8). Direct visual inspection of TLC sheets can detect gross contamination at levels of 2% DEG in acetaminophen elixirs and 6% DEG in glycerin. The assay costs $1.00 or less per test, can be performed without laboratory facilities, and takes approximately 20 minutes. Although detection limits of 0.1% using TLC methods require more sophisticated equipment, these low-cost methods would have detected contamination and likely prevented many of the fatalities in this event.
Because DEG poisonings continue to occur, in 2000 the World Health Organization (WHO) introduced the first global training program for industry personnel on safe manufacturing practices.§ In 2006, the International Medical Products Anti-Counterfeiting Taskforce was launched to strengthen regulatory enforcement and communication within and among countries.¶ In 2008, a new monograph on the safe manufacturing of oral liquid preparations was added to The International Pharmacopoeia, in response to several DEG poisoning events involving liquid medications.** Globalization of pharmaceutical manufacturing and distribution has heightened the need for more uniform regulation and international cooperation. These measures address specific vulnerabilities in the production, inspection, and distribution of pharmaceuticals internationally. Countries that inadequately implement safe manufacturing standards, poorly enforce quality controls, or lack adequate training programs remain at risk for medication-associated poisonings.
Acknowledgment
The findings in this report are based, in part, on contributions by J Schier, National Center for Environmental Health, CDC.
References
- Geiling EMK, Cannon PR. Pathologic effects of elixir of sulfanilamide (diethylene glycol) poisoning: a clinical and experimental correlation: final report. JAMA 1938;111:919--26.
- Okuonghae HO, Ighogboja IS, Lawson JO, Nwana EJ. Diethylene glycol poisoning in Nigerian children. Ann Trop Paediatr 1992;12:235--8.
- Rentz ED, Lewis L, Mujica OJ, et al. Outbreak of acute renal failure in Panama in 2006: a case-control study. Bull World Health Organ 2008;86:749--56.
- World Health Organization. Counterfeit drugs kill! IMPACT -- International Medical Products Anti-Counterfeiting Task Force. Geneva, Switzerland: World Health Organization; 2008. Available at http://www.who.int/entity/impact/finalbrochurewha2008a.pdf. Accessed December 4, 2009.
- Food and Drug Administration. Report on FDA's approach to medical product supply chain safety. Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration; 2009. Available at http://www.fda.gov/downloads/safety/safetyofspecificproducts/ucm184049.pdf. Accessed December 4, 2009.
- Food and Drug Administration. Guidance for industry: testing of glycerin for diethylene glycol. Rockville, MD: US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research; 2007. Available at http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm070347.pdf. Accessed December 1, 2009.
- Schier J, Rubin C, Miller D, Barr D, McGeehin M. Medication-associated diethylene glycol mass poisoning: a review and discussion on the origin of contamination. J Public Health Policy 2009;30:127--43.
- Kenyon AS, Xiaoye S, Yan W, Har NW. Simple, at-site detection of diethylene glycol/ethylene glycol contamination of glycerin and glycerin-base raw materials by thin-layer chromatography. J AOAC Int 1998;81:44--50.
* One parent estimated the onset of oliguria or anuria occurred 1 day before exposure to the medication, which might reflect the difficulty of recalling precise use of over-the-counter medications.
† DEG is a clear, colorless, odorless, mildly sweet liquid, and an efficient solvent for water-insoluble active ingredients in medications. DEG is readily absorbed orally and transdermally. Although a safe level has not been established in humans, a safety limit of 0.1% DEG for screening substances used to manufacture pharmaceutical products (e.g., active ingredients and excipients), was set by the United States Pharmacopeia, the official standards authority for health products sold in the United States. Data from prior outbreaks suggest that the minimum toxic dose is <1 mL/kg. Although the mechanism for toxicity is still unclear, 2-hydroxyethoxyacetic acid, the metabolic product of the enzyme aldehyde dehydrogenase, is considered to be a renal toxin.
§ Additional information available at http://www.who.int/medicines/areas/quality_safety/quality_assurance/production/en/index.html.
¶ Additional information available at http://www.who.int/impact/en.
** Available at http://www.who.int/medicines/publications/pharmacopoeia/overview/en/index.html.
|
What is already known on this topic? Large-scale poisonings resulting from medications contaminated with diethylene glycol (DEG) are a recurrent global public health problem. What is added by this report? During manufacturing, a liquid acetaminophen-based teething medication was contaminated with DEG, resulting in acute renal failure in 57 infants and toddlers in three Nigerian states, 54 of whom died. What are the implications for public health practice? Well-developed and strictly enforced pharmaceutical quality control measures and training programs can prevent DEG-associated large-scale poisoning events. |
FIGURE. Number of unexplained acute renal failure cases* (N = 56†), by onset date --- Nigeria, October 2008--February 2009
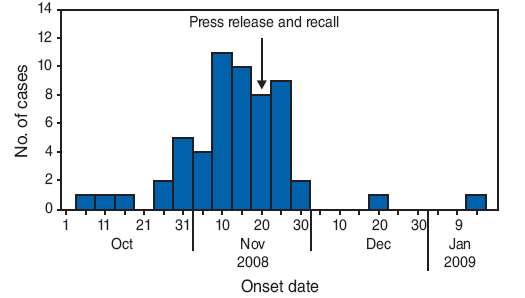
* An unexplained case of acute renal failure was defined as acute-onset anuria or oliguria of unknown etiology lasting ≥24 hours, with onset after August 1, 2008.
† A total of 57 patients met the case definition, but the onset date was unknown for one patient.
Alternate Text: The figure above shows the number of unexplained acute renal failure cases (N = 56), by onset date in Nigeria from October 2008 to February 2009. On November 18, 2008, the Nigerian Federal Ministry of Health (FMOH) received a report of 13 cases of unexplained acute renal failure among children from a hospital in Lagos state. Several of the ill patients had been exposed to a liquid acetaminophen-based teething medication, which became the focus of investigation. All the cases had occurred in children aged ≤3 years. Hospitals in Kaduna and Osun states reported similar clusters of ARF. On November 21, after NAFDAC officials discovered DEG contamination in batches of the medication, a full product recall was initiated, the manufacturing facility was shut down, and an investigation of the cases was conducted.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services. |
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 12/9/2009


