Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Swine-Origin Influenza A (H1N1) Virus Infections in a School --- New York City, April 2009
On April 24, 2009, CDC reported eight confirmed cases of swine-origin influenza A (H1N1) virus (S-OIV) infection in Texas and California (1). The strain identified in U.S. patients was confirmed by CDC as genetically similar to viruses subsequently isolated from patients in Mexico (1). Since April 24, the number of cases in the United States* and elsewhere† has continued to rise. As of April 28, approximately half (45) of all U.S. cases of S-OIV infection had been confirmed among students and staff members at a New York City (NYC) high school. This report describes the initial outbreak investigation by the NYC Department of Health and Mental Hygiene (DOHMH) and provides preliminary details about 44 of the 45 patients (the remaining patient resides outside of NYC and was not included in the analysis). The preliminary findings from this investigation indicate that symptoms in these patients appear to be similar to those of seasonal influenza. DOHMH will continue monitoring for changes in the epidemiology and/or clinical severity of S-OIV infection.
Epidemiologic and Laboratory Investigations
On April 23, DOHMH was notified of approximately 100 cases of mild (uncomplicated) respiratory illness among students at an NYC school (high school A) with 2,686 students and 228 staff members. During April 23--24, a total of 222 students visited the school nursing office and left school because of illness. Given initial reports on April 24 of what was later determined to be a large S-OIV outbreak in Mexico, DOHMH decided to rapidly mobilize staff members to go to high school A to collect nasopharyngeal swabs from any symptomatic students. On April 24 (a Friday), DOHMH staff members collected nasopharyngeal swabs from five newly symptomatic students identified by the school nurse and four newly symptomatic students identified at a nearby physician's office. A decision was made over the weekend not to open the school on Monday. Because of suspicion that the respiratory disease cases might be caused by S-OIV, beginning April 24, DOHMH attempted to contact the remaining 213 students reported by the nursing office to have left school because of respiratory illness. Some of the most recently symptomatic at the time of telephone contact were advised to visit a specified emergency department for nasopharyngeal swab collection. DOHMH also provided nasopharyngeal test kits to selected physicians' offices in the vicinity of high school A for collection of specimens from symptomatic staff members or students. On April 26, seven of the nine specimens collected on April 24 by DOHMH were identified by CDC as S-OIV. During April 26--28, 37 (88%) of 42 specimens collected in the emergency department and local physicians' offices tested positive at CDC for S-OIV, bringing the total number of confirmed cases to 44.
DOHMH conducted telephone interviews with the 44 patients with confirmed S-OIV on April 27. Median age of the patients was 15 years (range: 14--21 years). All were students, with the exception of one student teacher aged 21 years. Thirty-one (70%) of the 44 were female. Thirty (68%) were non-Hispanic white; seven (16%) were Hispanic; two (5%) were non-Hispanic black; and five (11%) were of other races. Four patients reported travel outside NYC within the United States in the week before symptom onset, and an additional patient traveled to Aruba in the 7 days before symptom onset. None of the 44 patients reported recent travel to California, Texas, or Mexico.
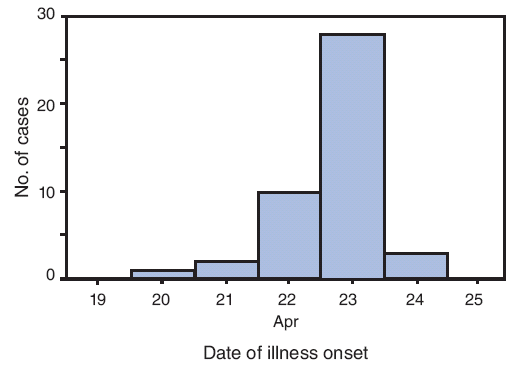
Illness onset dates ranged from April 20 to April 24; 10 (23%) of the patients had illness onset on April 22, and 28 (64%) had illness onset on April 23 (Figure). The most frequently reported symptoms were cough (in 43 patients [98%]), subjective fever (42 [96%]), fatigue (39 [89%]), headache (36 [82%]), sore throat (36 [82%]), runny nose (36 [82%]), chills (35 [80%]), and muscle aches (35 [80%]). Nausea (24 [55%]), stomach ache (22 [50%]), diarrhea (21 [48%]), shortness of breath (21 [48%]), and joint pain (20 [46%]) were less frequently reported but still common. Among 35 patients who reported a maximum temperature, the mean was 102.2°F (39.0°C) (range: 99.0--104.0°F [37.2--40.0°C]). In total, 42 (95%) patients reported subjective fever plus cough and/or sore throat, meeting the CDC definition for influenza-like illness (ILI) (2). At the time of interview on April 27, 37 patients (84%) reported that their symptoms were stable or improving, three (7%) reported worsening symptoms (two of whom later reported improvement), and four (9%) reported complete resolution of symptoms. Only one reported having been hospitalized for syncope and released after overnight observation.
Enhanced Surveillance
On April 26, DOHMH launched enhanced surveillance for self-reported ILI among all students, staff members, and family members of persons at high school A via an online survey. Students and staff members were recruited via e-mail messages with a link to the survey, followed by daily reminder e-mails. Active surveillance at the school was impractical because a decision was made by DOHMH and the school principal not to reopen the school for the start of the new school week, April 27. Complete data from this ongoing survey are not yet available, but preliminary results indicate widespread influenza-like symptoms, with hundreds of students and many staff members reporting symptoms that met the case definition for ILI. Several students participating in the on-line survey (none of whom had confirmed S-OIV) reported travel to Mexico during the week before April 20; an undetermined number were symptomatic at the time of survey participation.
DOHMH also initiated active surveillance for severe, hospitalized febrile respiratory ILI among NYC residents, and this surveillance is currently ongoing. On April 26, DOHMH staff members began contacting all 61 NYC hospitals with medical and/or pediatric intensive care units by telephone on a daily basis to identify possible severe cases of S-OIV, defined by the presence of fever ≥100.4°F (≥38°C) and at least one of the following: acute respiratory distress syndrome, pneumonia, or respiratory distress. DOHMH physicians review all possible cases; nasopharyngeal swabs are recommended for cases with no identified etiology. Specimens are tested for influenza A at the NYC Public Health Laboratory, and isolates that cannot be subtyped are sent to CDC for further characterization. Active surveillance identified one to two cases of severe hospitalized ILI per day for which further testing was recommended. Results of the testing are not yet available.
Enhanced passive surveillance also is ongoing. Doctors are asked via daily reminders on the Health Alert Network to report any hospitalized patients with fever and unexplained pneumonia or respiratory distress to DOHMH. All case reports are reviewed by DOHMH physicians, who contact providers reporting cases of severe illness consistent with possible swine influenza and arrange nasopharyngeal testing if warranted. In addition, DOHMH conducts syndromic surveillance for the following: emergency department visits for fever or influenza-like illness; drug sales for oseltamivir and other prescription drugs for influenza; and school absenteeism.
Reported by: HT Jordan, MD, MC Mosquera, MD; Swine Flu Investigation Team, New York City Dept of Health and Mental Hygiene, New York. H Nair, PhD, AM France PhD, EIS officers, CDC.
Editorial Note:
To date, this school-based outbreak is the largest cluster of S-OIV cases reported in the United States (2). The findings from this investigation (in a population known to be at low risk for severe disease from seasonal influenza) indicate that symptoms appear to be similar to those of seasonal influenza (3). The risk for severe disease among higher risk groups is not yet known. Additional assessment of the extent of illness in NYC is ongoing.
In crafting a local response to S-OIV, DOHMH has relied upon several years of pandemic preparedness planning, adapted to the specific characteristics of the current outbreak in NYC. Given the spectrum of disease observed thus far in NYC, DOHMH has given highest priority to active surveillance for severe illness in order to assure DOHMH's ability to rapidly detect any change in the virulence or epidemiology of the virus that would prompt consideration of changes in current policy regarding use of antivirals and community control measures. This decision also was influenced by the need to prioritize use of the public health laboratory's resources on testing those cases with clinical or epidemiologic characteristics that, if confirmed to be S-OIV, might influence a change in the DOHMH's recommendations for public health control measures. DOHMH's current primary goals are to assess the severity of disease in infected persons and to maintain the ability to detect changes in the epidemiology and clinical presentation of the virus. Aggressive containment in NYC is not a feasible strategy because the virus originated outside NYC and has been reported in multiple other locales.
At this time, NYC health-care providers have been advised by DOHMH to report all patients with severe, unexplained febrile respiratory illness, and to report patients with mild (uncomplicated) cases of ILI only if they are associated with a cluster of illness (i.e., three or more cases of ILI) in an institution. NYC providers have been advised to test patients with severe, unexplained febrile respiratory illnesses for influenza A but not to test patients with mild (uncomplicated) ILI unless they have conditions that increase their risk for more severe illness (3). DOHMH is recommending treatment with oseltamivir or zanamivir for 1) hospitalized persons with suspected, probable, or confirmed illness, or with severe febrile unexplained respiratory illness pending testing for swine influenza, or 2) patients with mild (uncomplicated) ILI and underlying conditions (e.g., chronic cardiovascular or renal disorders or immunosuppression) that increase the risk for more severe illness because of influenza. DOHMH is recommending treatment for any patient with mild (uncomplicated) ILI permissively only if started within 48 hours of symptom onset. Antiviral prophylaxis is being recommended for 1) health-care workers who provided care to patients with suspected, probable or confirmed swine influenza without using appropriate personal protection or 2) asymptomatic household or other close contacts of ill persons of suspected, probable, or confirmed swine influenza who are at higher risk for complications of influenza or are health-care workers themselves. Persons with mild (uncomplicated) ILI are being advised to stay home for 7 days after symptom onset or 24--48 hours after symptom resolution, whichever is longer, and to cover their coughs and sneezes and wash their hands frequently. But neither testing nor presumptive antiviral therapy are currently recommended for these persons. Guidance for health-care providers is available via the DOHMH Health Alert Network at http://www.nyc.gov/health/nycmed. Additional information from DOHMH on swine influenza is available at http://www.nyc.gov/health and http://www.nyc.gov/html/doh/downloads/pdf/cd/swine_flu_faq.pdf.
Interim guidance from CDC on treatment and chemoprophylaxis for swine influenza is available at http://www.cdc.gov/flu/swine/recommendations.htm. Interim guidance on infection control for swine influenza is available at http://www.cdc.gov/swineflu/guidelines_infection_control.htm. Additional information about swine influenza is available at http://www.cdc.gov/flu/swine/index.htm.
References
- CDC. Swine influenza A (H1N1) infections---California and Texas, April 2009. MMWR 2009;58:437--9.
- CDC. Update: infections with a swine-origin influenza A (H1N1) virus---United States and other countries, April 28, 2009. MMWR 2009;58:433--4.
- CDC. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR 2008;57(No. RR-7).