FIGURE. Number of confirmed cases of 2009 pandemic influenza A (H1N1) virus infection (N = 78),* by rank and date patient reported to the ship's infirmary, during an outbreak on a Peruvian Navy ship --- June--July 2009
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Outbreak of 2009 Pandemic Influenza A (H1N1) on a Peruvian Navy Ship --- June--July 2009
On June 25, 2009, a naval cadet reported to the infirmary of a 355-crewman Peruvian Navy ship with a febrile acute respiratory infection (FARI) 5 days after the ship docked in San Francisco, California. Pandemic 2009 influenza A (H1N1) virus was suspected as the cause because it was circulating in the city at that time. A test for pandemic H1N1 by real-time reverse transcription--polymerase chain reaction (rRT-PCR) was positive. During the subsequent 3 weeks, as the ship continued its cruise, 77 additional crew members developed confirmed pandemic H1N1 influenza. The U.S. Naval Medical Research Center Detachment (NMRCD), in collaboration with the Peruvian Navy, conducted an investigation to describe the outbreak and determine the attack rate for pandemic H1N1 influenza on the ship. This report summarizes the results of that investigation, which indicated that, of the 85 patients with FARI, 78 (92%) tested positive for pandemic H1N1 by rRT-PCR. The attack rate for confirmed pandemic H1N1 influenza was 22.0%. The most frequent symptoms, other than fever, were cough, headache, nasal congestion, and malaise. No complications or deaths occurred. Patients were treated according to World Health Organization (WHO) influenza treatment guidelines*; six patients received antiviral medication because of preexisting comorbidities. A shipboard respiratory surveillance program, which had been implemented aboard the ship before its departure from Peru, permitted the early detection of the outbreak. Subsequent implementation of control measures might have slowed the outbreak. Laboratory disease surveillance and adequate outbreak control procedures might reduce transmission of pandemic H1N1 influenza aboard ships.
Since 2002, the Peruvian Navy training ship ATC 131 has been making trips with second and fourth-year Peruvian Navy cadets visiting many ports of the world. In May 2009, the ship cruised from Peru to San Francisco via Ecuador and Costa Rica, stopped in San Francisco, (docked in port during June 20--24), and returned to Peru via Mexico (July 1--5) and Panama (July 10--12). In each port, the crew went ashore for protocol or visiting activities. Before the ship departed Peru, the crowded living conditions and difficulties in maintaining hygiene aboard ship prompted the Peruvian Navy to implement a respiratory surveillance program. Health personnel were trained on FARI diagnosis (oral temperature ≥100.5°F [≥38.1°C] and cough or sore throat) and respiratory swab specimen collection techniques. In addition, crew members were encouraged to seek medical attention through the ship's infirmary as soon as they developed signs or symptoms of respiratory illness (e.g., fever, cough, or sore throat). Personnel were provided with personal protective equipment (PPE) and were trained in proper respiratory hygiene.
Six weeks after departure, on June 25, 2009, 1 day after the ship set sail from San Francisco, one crew member reported to the infirmary with a 2-day history of fever of 101.3°F (38.5°C), sore throat, nasal congestion, headache, malaise, and cough after at least a 1-day visit ashore in San Francisco. After undergoing a negative rapid influenza test, the patient was discharged from the infirmary with symptomatic treatment but was not placed in isolation. Two days later, on June 27, another crew member reported to the infirmary with similar symptoms that had begun 1 day before, including a temperature of 102.9°F (39.4°C); however, he tested positive for influenza A with the rapid test. This second patient shared living quarters with the first patient. The first patient was then retested with a rapid test and was found to be positive for influenza A (Figure).† The two patients were placed in isolation and given symptomatic medication. This incident alerted the staff on board to a possible pandemic H1N1 outbreak.
During June 28--July 4, during the stopover in Mexico, 33 additional crew members reported to the infirmary with FARI symptoms. The first six underwent respiratory swab testing, and all six swabs tested positive for pandemic H1N1 using rRT-PCR by local health port authorities in Mexico. The other patients were presumed to have pandemic H1N1 infection. A case definition was then instituted. A case of pandemic H1N1 influenza was defined as illness in a person with FARI symptoms and laboratory-confirmed H1N1 infection by rRT-PCR. All respiratory swab samples from patients with FARI symptoms were then tested for pandemic H1N1 influenza by rRT-PCR and viral isolation at NMRCD after the ship had returned to Peru on July 17.
All subsequent patients with FARI symptoms had specimens tested for pandemic H1N1 influenza by rRT-PCR and viral isolation. The specimens were stored frozen in liquid nitrogen (at approximately -180ºC) until they were tested at NMRCD after the ship returned to Peru on July 17.
During July 5--11, an additional 41 crew members reported to the infirmary with FARI symptoms. An additional deck, adjacent to the infirmary, was made available for patient isolation. Patients were isolated for a minimum of 7 days (range: 7--9 days) or until symptoms resolved. All patients were given masks to help prevent them from spreading the virus to susceptible persons and were required to wear the mask for at least 5 days after discharge from the isolation facility. In addition to being recommended water and soap hygiene, all patients were provided with alcohol-based hand gel sanitizers to help reduce respiratory illness transmission (1). All remaining crew members were actively screened daily for FARI through a clinician-patient interview and by taking their oral temperatures; those who had at least one respiratory symptom were placed in isolation, given hand sanitizers and masks, and were monitored daily for additional symptoms. Upon docking in Panama on July 10, all onboard personnel were re-instructed in proper respiratory hygiene and given additional PPE. The following week, after departing from Panama, nine additional FARI cases were detected. The last case detected on the ship was in a patient who reported to the infirmary on July 16. All respiratory swab samples were stored in liquid nitrogen in the infirmary until they could be tested later at NMRCD laboratories.
Among 355 crew members, a total of 78 cases of pandemic H1N1 were confirmed by rRT-PCR. The attack rate was 22.0% (78 of 355) (Table). Respiratory swab specimens from seven patients with FARI tested negative for pandemic H1N1 by rRT-PCR. Attack rates varied by rank and age group (p<0.001, by chi-square and Fisher's exact test, respectively), with the highest values among cadets (31.4%), low-rank officers (i.e., warrant officers, petty officers, and enlisted personnel) (14.3%), and persons aged 18--25 years (30.1%). No difference in attack rates was observed between males and females (p=0.838, by chi-square test).
The mean age of patients with laboratory-confirmed cases was 25.5 years (range: 17.1--33.9 years), which was not different from that of the asymptomatic crew (p=0.051, by t-test). The mean temperature was 101.5°F (38.6°C) (range: 100.6--102.4°F [38.1--39.1°C]); mean number of days between onset of symptoms and presentation to the infirmary was 1.6 days (range: 0.8--2.4 days). The most frequent symptoms included cough and headache (both 75%), malaise (74%), nasal congestion (73%), and sore throat (55%); 99% of patients had been vaccinated against seasonal influenza (Agrippal S-1 inactivated subunit influenza vaccine, types A and B) before deployment. No complications or deaths occurred. Of the 78 patients, six received oseltamivir (75 mg twice daily) based on risk factor assessment and WHO treatment guidelines.
Reported by
DM Vera, MD, V Gonzaga, MSc, RA Hora, MD, M Ramos, MD, C Loret de Mola, MD, JM Neyra, MD, C Sanchez, MD, T Kochel, PhD, MJ Sklar, MD, JM Montgomery, PhD, US Naval Medical Research Center Detachment; JA Quispe, F Bringas, MD, M Céspedes, MD, S González, MD, M Larru, DDS, M Fernández, MD, Peruvian Navy Hospital (CEMENA), Lima, Peru. D Faix, C Meyers, P Blair, Naval Health Research Center, San Diego, California. P Mote, Dept of Biological Sciences, Franklin College of Arts and Sciences, Univ of Georgia. DL Blazes, Div of Global Emerging Infections Surveillance and Response System, Armed Forces Health Surveillance Center, US Department of Defense. T Quandelacy, Div of Viral and Rickettsial Diseases, National Center for Zoonotic, Vector-Borne, and Enteric Diseases, CDC.
Editorial Note
The shipboard pandemic H1N1 influenza outbreak described in this report likely began on June 25, 2009, 5 days after the ship docked in San Francisco. At the time the ship was docked in San Francisco, pandemic H1N1 was circulating throughout the city, and several infected patients might have been simultaneously exposed to infected persons ashore. Shipboard personnel have been known to acquire respiratory illnesses while in port, with subsequent spread to susceptible shipmates (2). The attack rate for the outbreak was 22.0%, somewhat lower than attack rates for influenza outbreaks in other similar, confined settings (37.0%--45.0%) (3,4) and lower than the attack rates in two other reported shipborne outbreaks of seasonal influenza (34.0%--77.0%) (2,5). Some of these previous outbreaks occurred aboard navy ships, among previously vaccinated crew members, and showed rapid spread of the virus in confined populations, despite appropriate vaccination. Although the majority of the crew members on the Peruvian ship were vaccinated against seasonal influenza, vaccination would not be expected to protect against pandemic H1N1. This result is not surprising and is consistent with previous findings.§ High influenza attack rates also have been described aboard passenger ships, where viral transmission is favored by close confinement (6). The relatively lower attack rate described in this outbreak might be explained by the early detection of the causative agent and the timely implementation of control measures by onboard health-care personnel.
The increased risk for disease transmission among low-rank personnel on navy ships has been observed previously (7). In this outbreak, the highest attack rates were among cadets and low-rank officers. Cadets had an attack rate twice that of low-rank officers, possibly because of the differences in living conditions aboard the ship. Bunk beds in cadet bunkers are closer together than those in low-rank officer bunkers, making possible higher disease transmission among cadets. Also, the physical proximity among crew members was higher during work hours among cadets and low-rank officers than among high-rank officers (i.e., junior, senior, and flag officers) and civilians.
A lag occurred between the onset of symptoms and presentation to the ship's infirmary, with a 2-day average. This might be explained by the propensity of crew members to intentionally neglect or hide their symptoms to avoid being placed in isolation in the infirmary while the ship is docked.
Crowding, rigorous working environments, physiologic stress, and the rapid transport of large numbers of persons provide ideal conditions for transmission and broad dissemination of respiratory disease pathogens (8). Because all of these are commonly occurring factors aboard naval ships, such populations might experience high rates of influenza illness during outbreaks (9). These findings highlight the importance of a robust respiratory surveillance system on board ships traveling to foreign ports. The findings also emphasize the crucial role of continuous surveillance for respiratory disease in the military because rapid detection is a major factor of successful intervention. Surveillance, particularly in these populations, can be extremely important for timely detection of outbreaks and adequate implementation of control measures, ultimately preventing potential dissemination back to their country of origin (10).
Acknowledgments
The findings in this report are based, in part, on contributions by M del Pilar Cabrera, MD, C Choncen, MD, H Dapello, DDS, N Cueva, J Rengifo, C Carhuamaca, S Castro, C Salcedo, and MA Esquivel, Peruvian Navy Health Directorate.
References
- Mott PJ, Sisk BW, Arbogast JW, Ferrazzano-Yaussy C, Bondi CA, Sheehan JJ. Alcohol-based instant hand sanitizer use in military settings: a prospective cohort study of Army basic trainees. Mil Med 2007;172:1170--6.
- Ksiazek TG, Olson JG, Irving GS, Settle CS, White R, Petrusso R. An influenza outbreak due to A/USSR/77-like (H1N1) virus aboard a US Navy ship. Am J Epidemiol 1980;112:487--94.
- Klontz KC, Hynes NA, Gunn RA, Wilder MH, Harmon MW, Kendal AP. An outbreak of influenza A/Taiwan/1/86 (H1N1) infections at a naval base and its association with airplane travel. Am J Epidemiol 1989;129:341--8.
- Makras P, Alexiou-Daniel S, Antoniadis A, Hatzigeorgiou D. Outbreak of meningococcal disease after an influenza B epidemic at a Hellenic Air Force recruit training center. Clin Infect Dis 2001;33:e48--50.
- Earhart KC, Beadle C, Miller LK, et al. Outbreak of influenza in highly vaccinated crew of U.S. Navy ship. Emerg Infect Dis 2001;7:463--5.
- Mouchtouri V, Black N, Nichols G, et al. Preparedness for the prevention and control of influenza outbreaks on passenger ships in the EU: the SHIPSAN TRAINET project communication. Euro Surveill 2009;14(21).
- Ziebold C, Hassenpflug B, Wegner-Brose H, Wegner K, Schmitt HJ. An outbreak of rubella aboard a ship of the German Navy. Infection 2003;31:136--42.
- Cross ER, Hermansen LA, Pugh WM, White MR, Hayes C, Hyams KC. Upper respiratory disease in deployed U.S. Navy shipboard personnel. Mil Med 1992;157:649--51.
- Kilbourne ED. The epidemiology of influenza. In: Influenza. New York, New York: Plenum Medical Book Co.; 1987.
- Green IJ, Hung SC, Irving GS, Davenport JV. Influenza A virus aboard a US Navy ship in 1972 and the antibody response of the crew to influenza vaccine. Mil Med 1975;140:179--81.
* Available at http://www.who.int/csr/resources/publications/influenza/WHO_CDS_CSR_RMD_2004_8/en/index.html.
† Specimens for both patients were tested using the QuickVue Influenza A+B test kit (Quidel Corporation, San Diego, California).
§ Available at http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5844a5.htm.
What is already known on this topic?
Influenza can disseminate rapidly within populations living in confined settings, causing considerable morbidity and loss of work days among young adults and disrupting daily activities affecting the preparedness of military units.
What is added by this report?
An outbreak of pandemic H1N1 influenza occurred on a Peruvian naval ship over a period of 3 weeks; the attack rate for laboratory-confirmed infection was 22.0%, and lower-ranking personnel had higher attack rates than higher-ranked personnel.
What are the implications for public health practice?
Laboratory disease surveillance and adequate outbreak response procedures, in conjunction with aggressively controlling respiratory illnesses within confined military settings, such as naval vessels, should be considered as part of health policies aimed at reducing the transmission of these infections.
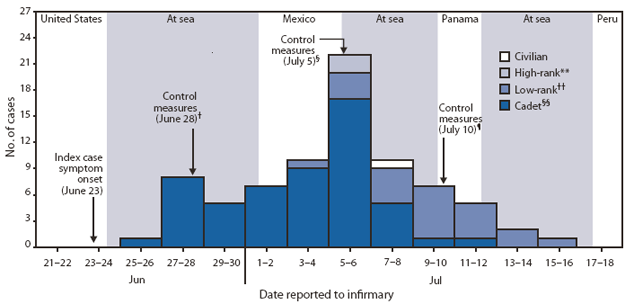
* A case of pandemic H1N1 influenza was defined as illness in a person with symptoms of febrile acute respiratory infection (FARI) and laboratory-confirmed H1N1 infection by real-time reverse transcription--polymerase chain reaction (rRT-PCR). Specimens from all patients with FARI symptoms were then tested for pandemic H1N1 influenza by rRT-PCR and viral isolation at the Naval Medical Research Center Detachment in Lima after the ship had returned to Peru on July 17. All specimens were kept in liquid nitrogen until tested.
† Patients were placed in isolation and given symptomatic medication.
§ Personnel were restricted regarding their movements on ship. An additional deck, adjacent to the infirmary, was made available for patient isolation. Patients were given masks, recommendations regarding water and soap hygiene, and alcohol-based hand gel sanitizers. All remaining crew members were screened daily for possible cases.
¶ All onboard personnel received additional biosafety training and materials, and were re-instructed in proper respiratory hygiene.
** Defined as junior, senior, and flag officers.
†† Defined as warrant officers, petty officers, and enlisted personnel.
§§ Defined as 2nd and 4th year trainee officers in the Peruvian Naval Academy.
Alternative Text: The figure above shows the number of confirmed cases of 2009 pandemic influenza A (H1N1) virus infection, by rank and date patient reported to the ship's infirmary, during an outbreak on a Peruvian Navy ship during June-July 2009. Six weeks after departure, on June 25, 2009, one crew member reported to the infirmary with a 2-day history of fever of 101.3°F (38.5°C), sore throat, nasal congestion, headache, malaise, and cough. After undergoing a negative rapid influenza test, the patient was discharged from the infirmary with symptomatic treatment but was not placed in isolation. Two days later, on June 27, another crew member reported to the infirmary with similar symptoms that had begun 1 day before, including a temperature of 102.9°F (39.4°C); however, he tested positive for influenza A with the rapid test. This second patient shared living quarters with the first patient. The first patient was then retested with a rapid test and was found to be positive for influenza A.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.
 ShareCompartir
ShareCompartir


