Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Trends in Smoking Before, During, and After Pregnancy --- Pregnancy Risk Assessment Monitoring System (PRAMS), United States, 31 Sites, 2000--2005
Abstract
Problem: Smoking among nonpregnant women contributes to reduced fertility, and smoking during pregnancy is associated with delivery of preterm infants, low infant birthweight, and increased infant mortality. After delivery, exposure to secondhand smoke can increase an infant's risk for respiratory tract infections and for dying of sudden infant death syndrome. During 2000--2004, an estimated 174,000 women in the United States died annually from smoking-attributable causes, and an estimated 776 infants died annually from causes attributed to maternal smoking during pregnancy.
Reporting Period Covered: 2000--2005.
Description of System: The Pregnancy Risk Assessment Monitoring System (PRAMS) was initiated in 1987 and is an ongoing state- and population-based surveillance system designed to monitor selected maternal behaviors and experiences that occur before, during, and after pregnancy among women who deliver live-born infants in the United States. Self-reported questionnaire data are linked to selected birth certificate data and are weighted to represent all women delivering live infants in the state. Self-reported smoking data were obtained from the PRAMS questionnaire and birth certificates. This report provides data on trends (aggregated and site-specific estimates) of smoking before, during, and after pregnancy and describes characteristics of female smokers during these periods.
Results: For the study period 2000--2005, data from 31 PRAMS sites (Alabama, Alaska, Arkansas, Colorado, Florida, Georgia, Hawaii, Illinois, Louisiana, Maine, Maryland, Michigan, Minnesota, Mississippi, Montana, Nebraska, New Jersey, New Mexico, New York, New York City, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Rhode Island, South Carolina, Utah, Vermont, Washington, and West Virginia) were included in this report. All 31 sites have met the Healthy People 2010 (HP 2010) objective of increasing the percentage of pregnant smokers who stop smoking during pregnancy to 30%; site-specific quit rates in 2005 ranged from 30.2% to 61.0%. However, none of the sites achieved the HP 2010 objective of reducing the prevalence of prenatal smoking to 1%; site-specific prevalence of smoking during pregnancy in 2005 ranged from 5.2% to 35.7%. During 2000--2005, two sites (New Mexico and Utah) experienced decreasing rates for smoking before, during, and after pregnancy, and two sites (Illinois and New Jersey) experienced decreasing rates during pregnancy only. Three sites (Louisiana, Ohio, and West Virginia) had increases in the rates for smoking before, during, and after pregnancy, and Arkansas had increases in rates before pregnancy only. For the majority of sites, smoking rates did not change over time before, during, or after pregnancy. For 16 sites (Alaska, Arkansas, Colorado, Florida, Hawaii, Illinois, Maine, Nebraska, New Mexico, New York [excluding New York City], North Carolina, Oklahoma, South Carolina, Utah, Washington, and West Virginia) for which data were available for the entire 6-year study period, the prevalence of smoking before pregnancy remained unchanged, with approximately one in five women (from 22.3% in 2000 to 21.5% in 2005) reporting smoking before pregnancy. The prevalence of smoking during pregnancy declined (p = 0.01) from 15.2% in 2000 to 13.8% in 2005, and the prevalence of smoking after delivery declined (p = 0.04) from 18.1% in 2000 to 16.4% in 2005.
Interpretation: The results indicate that efforts to reduce smoking prevalence among female smokers before pregnancy have not been effective; however, efforts targeting pregnant women have met some success as rates have declined during pregnancy and after delivery. Current tobacco-control efforts and smoking-cessation efforts targeting pregnant women are not sufficient to reach the HP 2010 objective of reducing prevalence of smoking during pregnancy.
Public Health Action: The data provided in this report are important for developing, monitoring, and evaluating state tobacco-control policies and programs to reduce smoking among female and pregnant smokers. States can reduce smoking before, during, and after pregnancy through sustained and comprehensive tobacco-control efforts (e.g., smoke-free policies and tobacco excise taxes). Health-care providers should increase efforts to assess the smoking status of their patients and offer effective smoking-cessation interventions to every female or pregnant smoker to whom they provide health-care services.
Introduction
Prenatal smoking remains one of the most common preventable causes of infant morbidity and mortality and is associated with 30% of small-for-gestational-age infants, 10% of preterm infants, and 5% of infant deaths (1,2). Cigarette smoking before conception can cause reduced fertility and conception delay among women (2,3). Maternal cigarette smoking during pregnancy increases the risk for pregnancy complications (e.g., placental previa, placental abruption, and premature rupture of the membrane) and poor pregnancy outcomes (e.g., preterm delivery, restricted fetal growth, and sudden infant death syndrome [SIDS]) (2,3). Exposure to secondhand smoke after delivery increases an infant's risk for respiratory tract infections (e.g., bronchitis and pneumonia), ear infections, and dying from SIDS (2--6). During 2000--2004, an estimated 174,000 women in the United States died annually from smoking-attributable causes, and an estimated 776 infants died annually from causes attributed to maternal smoking during pregnancy (7).
In 2005, approximately 10%--12% of women giving birth reported smoking during pregnancy based on birth certificates (8). Rates were higher among certain subpopulations (e.g., women aged <25 years, women with lower levels of education, American Indian/Alaska Native women, and white women) (8). Although smoking rates among women have been decreasing in the United States, an estimated 22% of women of reproductive age continued to smoke in 2006 (9).
Two Healthy People 2010 (HP 2010) national health objectives address smoking during pregnancy: 1) reduce the prevalence of cigarette smoking among pregnant women to 1% (objective no. 16-17) and 2) increase the percentage of pregnant smokers who stop smoking during pregnancy to 30% (objective no. 27-6) (10). A previous study of 10 states (Alabama, Alaska, Florida, Georgia, Maine, New York [excluding New York City*], Oklahoma, South Carolina, Washington, and West Virginia) that participated in the Pregnancy Risk Assessment Monitoring System (PRAMS) indicated that quit rates during pregnancy increased from 37.0% in 1993 to 46.0% in 1999, but no change occurred in smoking rates before pregnancy or in postpartum relapse rates during the study period (11). In 2003, 19 PRAMS sites (Alabama, Alaska, Arkansas, Colorado, Florida, Hawaii, Illinois, Louisiana, Maine, Nebraska, New Mexico, New York [excluding New York City], North Carolina, Ohio, Oklahoma, South Carolina, Utah, Washington, and West Virginia) achieved the second objective, with quit rates ranging from 30.2% in West Virginia to 65.8% in Utah (12). However, none of the sites achieved the first objective, with prevalence of smoking during the last 3 months of pregnancy ranging from 3.9% in Utah to 27.5% in West Virginia.
To assess progress toward achieving the HP 2010 objectives and to assist tobacco-control efforts targeting nonpregnant, pregnant, and postpartum female smokers, CDC analyzed data from the PRAMS questionnaire for 2000--2005. This report provides the first summary of PRAMS data concerning trends (aggregated and site-specific estimates) of smoking before, during, and after pregnancy and describes characteristics of female smokers during these periods.
Methods
Project Description
Initiated in 1987, PRAMS is an ongoing state- and population-based surveillance system designed to monitor selected self-reported behaviors, health-care use, and maternal morbidities that occur before, during, and after pregnancy among women who deliver a live-born infant in the United States. PRAMS is administered by CDC's National Center for Chronic Disease Prevention and Health Promotion in collaboration with state health departments. During 1987--2008, PRAMS began with six sites (District of Columbia, Indiana, Maine, Michigan, Oklahoma, and West Virginia) and now includes 37 states (Alabama, Alaska, Arkansas, Colorado, Delaware, Florida, Georgia, Hawaii, Illinois, Louisiana, Maine, Maryland, Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Nebraska, New Jersey, New Mexico, New York, North Carolina, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Utah, Vermont, Virginia, Washington, West Virginia, Wisconsin, and Wyoming), a tribal-state collaborative project (South Dakota), and one major metropolitan city (New York City).
Data Collection
All health departments participating in PRAMS use a standardized data collection methodology developed by CDC (13). At each site, a monthly stratified sample of 100--300 new mothers is selected systematically from recent birth certificates. PRAMS staff at each site mail a self-administered questionnaire to the selected women starting 2--3 months after the delivery of a live infant. Women who do not respond to any of three serial mailings are contacted by telephone to complete the survey. To minimize recall bias, all efforts to contact women by mail and telephone end 9 months postpartum. Survey data are linked to selected birth certificate data and weighted for sample design, nonresponse, and noncoverage.
The PRAMS questionnaire is revised periodically. Data highlighted in this report were collected using the Phase Four (2000--2003) and Phase Five (2004--2005) versions of the questionnaire (14). Details concerning the PRAMS methodology have been described elsewhere (13).
PRAMS sites were included in the report if an overall weighted response rate of ≥70% was achieved for a given year for every site. To minimize nonresponse bias, PRAMS has established 70% as the minimum weighted response rate for site data to be included in published results. The weighted response rate indicates the proportion of women sampled who completed a survey, adjusting for sample design.
For the study period 2000--2005, data from 31 PRAMS sites (Alabama, Alaska, Arkansas, Colorado, Florida, Georgia, Hawaii, Illinois, Louisiana, Maine, Maryland, Michigan, Minnesota, Mississippi, Montana, Nebraska, New Jersey, New Mexico, New York, New York City, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Rhode Island, South Carolina, Utah, Vermont, Washington, and West Virginia) were included in this report. Data from 26 PRAMS sites (Alaska, Arkansas, Colorado, Florida, Georgia, Hawaii, Illinois, Maine, Maryland, Michigan, Minnesota, Nebraska, New Jersey, New Mexico, New York, New York City, North Carolina, Ohio, Oklahoma, Oregon, Rhode Island, South Carolina, Utah, Vermont, Washington, and West Virginia) with data available in 2005 were aggregated for the analysis of maternal characteristics associated with smoking, and data from 16 PRAMS sites (Alaska, Arkansas, Colorado, Florida, Hawaii, Illinois, Maine, Nebraska, New Mexico, New York [excluding New York City], North Carolina, Oklahoma, South Carolina, Utah, Washington, and West Virginia) with data available for all 6 years during 2000--2005 were aggregated to analyze time trends in the prevalence of smoking. The 31 PRAMS sites included in this report represent approximately 54% of live births in the United States during 2005.
Data Analysis
This report presents data on five measures of smoking behaviors before, during, and after pregnancy: 1) smoking during the 3 months before pregnancy, 2) smoking during pregnancy (reported by women on the PRAMS questionnaire for the last 3 months of pregnancy or indicated on the linked birth certificate at any time during pregnancy†), 3) quitting smoking during pregnancy among women who smoked before pregnancy, 4) smoking after delivery (measured at the time the questionnaire was completed), and 5) relapsing to smoking after delivery among women who quit smoking during pregnancy (see Appendix for definitions). Three PRAMS questions were used to calculate these measures: 1) "In the 3 months before you got pregnant, how many cigarettes did you smoke on an average day?"; 2) "In the last 3 months of your pregnancy, how many cigarettes did you smoke on an average day?"; and 3) "How many cigarettes do you smoke on an average day now?"
Smoking measures were analyzed in the aggregate by selected maternal characteristics for the 26 sites with available data for 2005. Maternal age, race/ethnicity, education, marital status, parity, and initiation of prenatal care were derived from the birth certificate. Annual income, prepregnancy body mass index, pregnancy intention, health-care insurance coverage during pregnancy, Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) enrollment during pregnancy, alcohol use during pregnancy, and number of cigarettes smoked per day before pregnancy were derived from the PRAMS questionnaire (see Appendix for variable definitions). Smoking prevalence and standard errors were estimated by year for each site and aggregated only for the 16 sites with available data for all 6 years during 2000--2005.
To estimate the magnitude of change in the prevalence estimates, the proportion change was calculated by subtracting the smoking prevalence of the most recent year available from that of the earliest year available and dividing the difference by the prevalence from the earliest year. Statistical linear trends were assessed using logistic regression with smoking as the outcome variable and the infant's birth year as the independent variable. Only sites with at least 3 years of data were assessed for linear trends. Chi-square tests for independence were conducted to test for differences in smoking and maternal characteristics for categorical variables. Significance level for all statistical tests was set at p≤0.05.
Data were weighted to represent all live births delivered in each respective site in the given year. The analyses were conducted using SUDAAN version 9.1 to account for the complex survey design of PRAMS (15).
Results
A total of 31 PRAMS sites that had ≥1 year of data available during 2000--2005 were included in the analysis, with a total unweighted sample size of 242,038. The yearly site-specific sample size ranged from 770 respondents in New York City in 2004 to 2,518 respondents in Hawaii in 2000. The highest (89%) 1-year response rate was achieved in Utah in 2004. The average age of infants for whom the PRAMS questionnaire was completed varied (range: 93.8 days [Montana in 2002]--146.7 days [Florida in 2005]) on the basis of the most recent year of data available for a site (Table 1).
Maternal Characteristics of Smokers and Nonsmokers
In 2005, on the basis of aggregated data from 26 sites, 22.5% of women reported smoking before or during pregnancy or after delivery (Table 2). Compared with nonsmokers, women who smoked were significantly more likely to be younger (aged <25 years), non-Hispanic white, have ≤12 years of education, be unmarried, have an annual income of <$15,000, be underweight, have an unintended pregnancy, be first-time mothers, have initiated prenatal care later, be Medicaid-enrolled, and be enrolled in WIC during pregnancy (Table 2).
Smoking Before Pregnancy
Site-Specific Trends
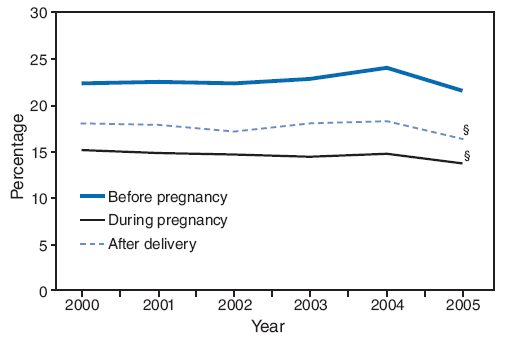
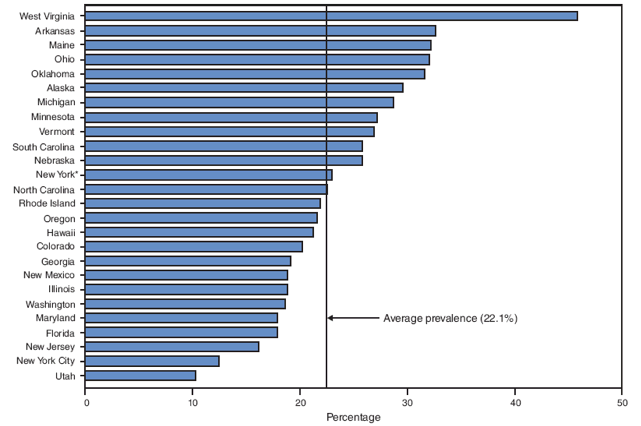
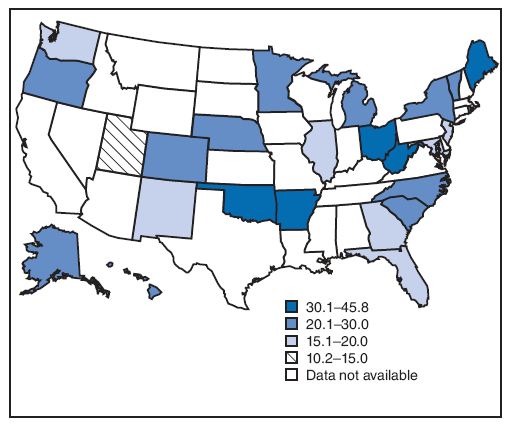
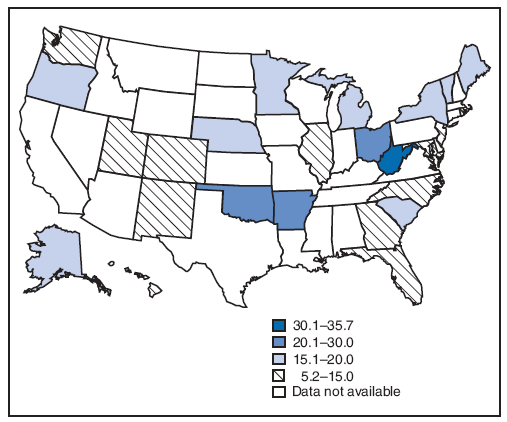
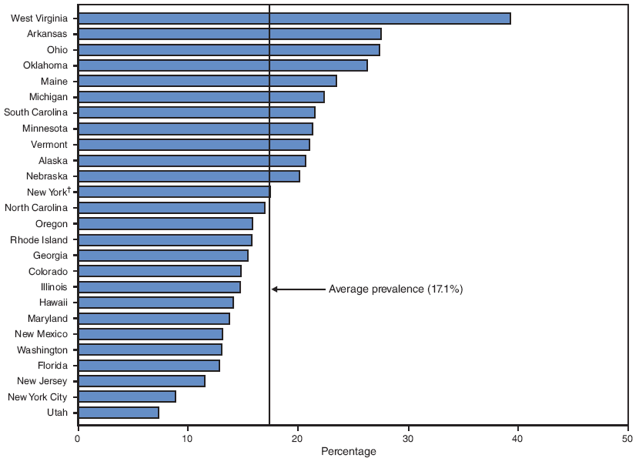
On the basis of aggregated data from the 16 sites for which data were available for all 6 years during 2000--2005, the prevalence of smoking during the 3 months before pregnancy did not change significantly (from 22.3% to 21.5%) (Figure 1; Table 3). During 2000--2005, the prevalence of smoking during the 3 months before pregnancy increased significantly for four sites (Arkansas [from 28.6% to 32.6%], Louisiana [from 21.2% to 28.2%], Ohio [from 28.6% to 32.0%], and West Virginia [from 36.2% to 45.8%]) and decreased significantly for two sites (New Mexico [from 21.3% to 18.8%] and Utah [from 14.3% to 10.2%]) (Table 3). In 2005, among 26 sites, site-specific prevalence of smoking during the 3 months before pregnancy ranged from 10.2% in Utah to 45.8% in West Virginia (Figures 2 and 3; Table 3).
Smoking During Pregnancy
Site-Specific Trends
PRAMS datasets include two sources for data on smoking during pregnancy: self-reported smoking during the last 3 months of pregnancy reported on the PRAMS questionnaire and smoking at any time during pregnancy indicated on the linked birth certificate. Estimates from each data source are presented with a combined estimate, which includes smoking during pregnancy indicated on either source. In 2005, on the basis of aggregated data from the 26 sites, the combined estimate for smoking during pregnancy (14.1%) was 16% higher than the PRAMS-only estimate (12.2%) and 38% higher than the birth certificate--only estimate (10.2%; data not presented). The prevalence of smoking during pregnancy based on the combined estimate was higher than the estimate based on either data source alone in all 31 sites.
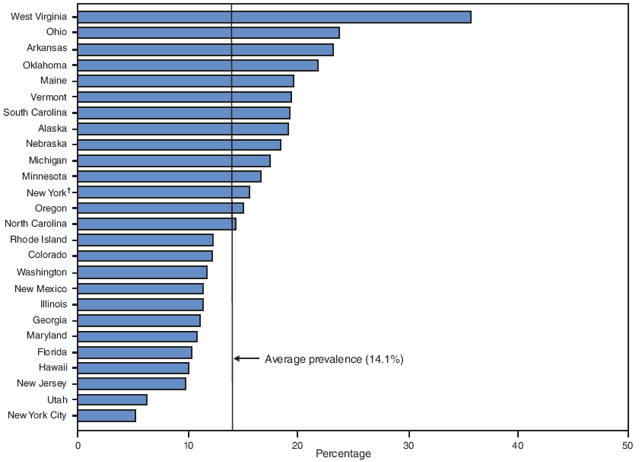
On the basis of aggregated data from the 16 sites, smoking during pregnancy (combined estimate) decreased significantly during 2000--2005, from 15.2% to 13.8%; however, this trend differed by site (Figure 1; Table 4). During the study period, smoking during pregnancy (combined estimate) increased significantly for three sites (Louisiana [from 13.7% to 18.9%], Ohio [from 20.2% to 23.8%], and West Virginia [from 29.4% to 35.7%] and decreased significantly for four sites (Illinois [from 14.6% to 11.3%], New Jersey [from 11.5% to 9.8%], New Mexico [from 13.1% to 11.3%], and Utah [from 9.9% to 6.2%]) (Table 4). The site-specific prevalence of smoking during pregnancy (combined estimate) among the 26 sites in 2005 ranged from 5.2% in New York City to 35.7% in West Virginia (Figures 4 and 5; Table 4).
Maternal Racial/Ethnic, and Age Trends
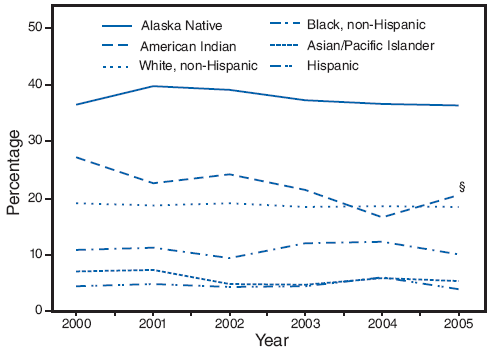
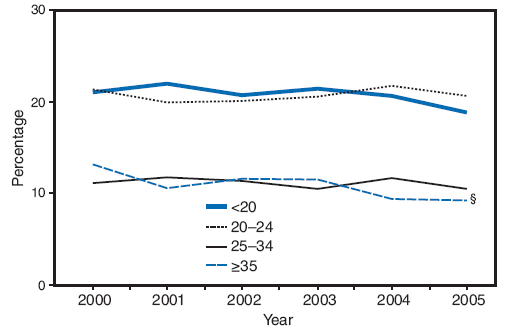
On the basis of aggregated data from the 16 sites with data available for all 6 years during 2000--2005, trends in smoking during pregnancy varied by maternal racial/ethnic and age categories. American Indians were the only racial/ethnic population that experienced a significant decline in smoking during pregnancy, from 27.1% in 2000 to 20.6% in 2005 (Figure 6; Table 5). In 2005, prevalence of smoking was highest among Alaska Natives (36.3%) and American Indians (20.6%) and lowest among Hispanics (4.0%) and Asian/Pacific Islanders (5.4%) (Table 5). Among age groups, only women aged ≥35 years had a significant decline in smoking during pregnancy, from 13.2% in 2000 to 9.2% in 2005 (Figure 7; Table 5). In 2005, the prevalence of smoking was highest among women aged 20--24 years (20.7%) and lowest among women aged ≥35 years (9.2%) (Table 5).
Breakdowns by age within racial/ethnic subpopulations demonstrated different trends over time. During 2000--2005, the prevalence of prenatal smoking increased significantly (from 30.0% to 32.8%) among non-Hispanic white women aged 20--24 years and decreased significantly (from 15.3% to 10.8%) among non-Hispanic white women aged ≥35 years (Table 5). Among non-Hispanic black women aged 25--34 years, the prevalence of smoking increased significantly (from 8.9% to 12.0%) (Table 5). Among Alaska Natives aged 20--24 years, the prevalence of prenatal smoking decreased significantly (from 44.8% to 36.1%) (Table 5). Among American Indians, the prevalence of prenatal smoking decreased significantly among women aged <20 years (from 37.6% to 15.9%) and among women aged 20--24 years (from 30.9% to 17.0%) (Table 5).
Quitting Smoking During Pregnancy
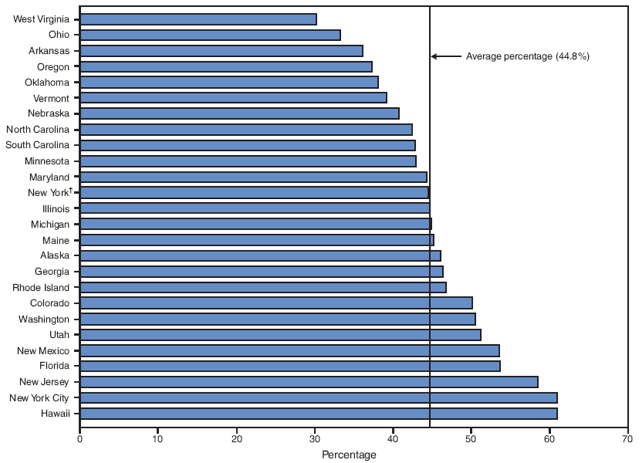
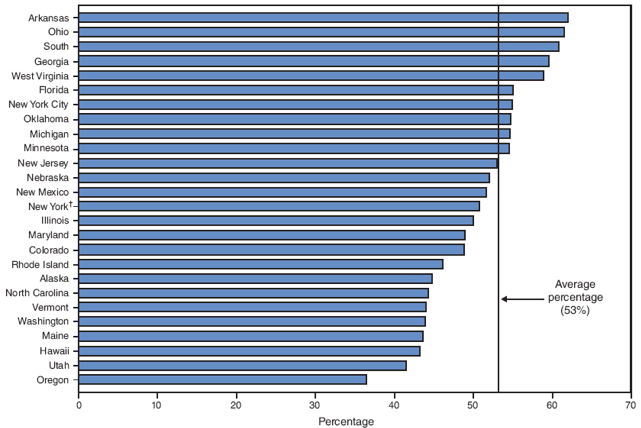
During the study period, on the basis of aggregated data from the 16 sites, the percentage of smokers who quit smoking during pregnancy did not increase significantly (from 43.9% to 45.7%) (Table 6). However, significant site-specific trends over time were observed. The percentage of smokers who quit smoking during pregnancy decreased significantly for one site (Louisiana [from 44.2% to 37.1%]) and increased significantly for two sites (Michigan [from 33.1% to 44.9%] and New Jersey [from 47.9% to 58.5%]) (Table 6). The site-specific percentage of women who quit smoking during pregnancy among the 26 sites in 2005 ranged from 30.2% in West Virginia to 61.0% in Hawaii (Figure 8; Table 6).
The 2005 aggregated data from 26 sites indicate that women who quit smoking during pregnancy differed from those who continued to smoke. Compared with women who did not quit smoking, women who quit were more likely to be Hispanic or Asian/Pacific Islander, had >12 years of education, were married, had an annual income of ≥$15,000, had an intended pregnancy, had normal or overweight body mass index, were first-time mothers, initiated prenatal care in the first trimester, were not Medicaid-enrolled, were not enrolled in WIC, did not use alcohol during pregnancy, and smoked fewer cigarettes before pregnancy (Table 7).
Smoking After Delivery
On the basis of aggregated data from the 16 sites, the prevalence of smoking after delivery decreased significantly (from 18.1% to 16.4%) during the study period (Figure 1; Table 8). The prevalence of smoking after delivery increased significantly for three sites (Louisiana [from 18.7% to 23.4%], Ohio [from 24.2% to 27.4%], and West Virginia [from 31.6% to 39.3%]) and decreased significantly for two sites (New Mexico [from 16.0% to 13.1%] and Utah [from 9.5% to 7.3%]) (Table 8). In 2005, the site-specific prevalence of smoking after delivery among the 26 states ranged from 7.3% in Utah to 39.3% in West Virginia (Figures 9 and 10; Table 8).
Relapsing to Smoking After Delivery
On the basis of aggregated data from the 16 sites, the percentage of women who relapsed to smoking after delivery was not statistically different (50.3% in 2000 compared with 51.4% in 2005) (Table 9). During the study period, the percentage of women relapsing to smoking after delivery increased significantly for three sites (Arkansas [from 43.1% to 62.0%], Minnesota [from 40.1% to 54.5%], and Vermont [from 34.7% to 44.0%]) (Table 9). In 2005, the percentage of women in the 26 sites who relapsed to smoking after delivery ranged from 36.4% in Oregon to 62.0% in Arkansas (Figure 11; Table 9).
Characteristics of women who quit smoking during pregnancy and did not start smoking again after delivery differed from those of women who relapsed to smoking. Compared with women who did not start smoking again after delivery, women who relapsed to smoking after delivery were significantly more likely to be aged <25 years, be non-Hispanic black, have <12 years of education, be unmarried, have an annual income of <$15,000, have had an unintended pregnancy, have entered prenatal care during or after the second trimester, have had Medicaid coverage, and be enrolled in WIC during pregnancy (Table 10).
Discussion
During 2000--2005, the overall aggregated prevalence of smoking before pregnancy for the 16 sites for which data were available for all 6 years remained unchanged, with approximately one in five women (from 22.3% in 2000 to 21.5% in 2005) reporting smoking during the 3 months before pregnancy. This finding suggests that efforts in the United States to prevent smoking and increase cessation among female smokers before becoming pregnant have not been effective. However, during 2000--2005, significant declines occurred in the prevalence of women smoking while pregnant (from 15.2% to 13.8%) and after delivery (from 18.1% to 16.4%), suggesting that efforts targeting pregnant and postpartum women have been effective. During 2000--2005, two sites (New Mexico and Utah) had decreases in the smoking prevalence before, during, and after pregnancy, and two sites (Illinois and New Jersey) had decreases in the smoking prevalence during pregnancy only. Three sites (Louisiana, Ohio, and West Virginia) had increases in the prevalence of smoking before, during, and after pregnancy, and Arkansas had increases in the smoking prevalence before pregnancy. For the majority of sites, smoking rates did not change over time before, during, or after pregnancy.
The 31 PRAMS sites included in this report have achieved the HP 2010 objective of increasing the percentage of pregnant smokers who stop smoking during pregnancy to 30%, although variations occurred across the PRAMS sites. In 2005, quit rates during pregnancy ranged from 30.2% in West Virginia to 61.0% in Hawaii. However, none of the sites achieved the HP 2010 objective of reducing prevalence of smoking during pregnancy to 1%; site-specific prevalence of smoking during pregnancy in 2005 ranged from 5.2% in New York City to 35.7% in West Virginia.
State tobacco-control efforts should be increased to achieve the HP 2010 objective of reducing prenatal smoking to 1%. Effective state and local strategies to prevent the initiation of smoking or to increase smoking cessation among nonpregnant women include banning all forms of tobacco advertisement, enforcing laws that prohibit sales to children and adolescents, promoting smoke-free policies in public places and in the workplace, and increasing taxes on cigarettes (16). A 10.0% increase in the price of tobacco products would result in an estimated 3.7% decrease in the number of adolescents who use tobacco and an estimated 4.1% decrease in the amount of tobacco used by the general population (16). Raising state tobacco taxes might assist states with high smoking rates and current low excise tax rates. In 2008, state tobacco excise taxes ranged from $0.07 to $2.75 per pack, and 25 states (Alabama, Arkansas, California, Colorado, Florida, Georgia, Idaho, Illinois, Indiana, Kansas, Kentucky, Louisiana, Mississippi, Missouri, Nebraska, Nevada, New Mexico, North Carolina, North Dakota, South Carolina, Tennessee, Utah, Virginia, West Virginia, and Wyoming) had excises taxes lower than $1.00 per pack (17).
Smoking cessation counseling and programs offered during prenatal care can provide effective assistance to encourage pregnant women to quit smoking, especially women who were light- to moderate-level smokers before becoming pregnant (i.e., women who smoke <1 pack of cigarettes a day) (18,19). However, the number of smokers who are offered such interventions is unknown. Studies of prenatal care providers in a few states indicate that approximately 30%--60% provide intensive interventions or referrals to cessation programs to pregnant smokers (20--22). Therefore, health-care providers can increase promotion and use of evidence-based cessation services. Each state has a telephone quitline that provides cessation counseling and support and is a free resource that can be promoted (e.g., through mass media campaigns and provider referrals) to all smokers (23).
Because higher rates of smoking during pregnancy were observed among Medicaid-enrolled women, comprehensive Medicaid coverage of tobacco-dependence treatments needs to be made available for all smokers who want to quit. As of 2006, seven state Medicaid programs (California, Indiana, Minnesota, New York, Oregon, Pennsylvania, and West Virginia) covered all forms of tobacco-dependence medications and at least one form of counseling, and 39 programs covered at least one form of treatment (24). However, even when coverage is available, providers and patients often are unaware of the Medicaid coverage. In a survey conducted in two states with comprehensive Medicaid coverage of cessation treatment, only 36% of Medicaid-enrolled smokers and 60% of Medicaid physicians knew that their state Medicaid program offered any coverage for tobacco-dependence treatments (25). States can encourage Medicaid coverage for tobacco-dependence treatments for all smokers and promote awareness of this coverage among providers and patients.
The results presented in this report indicate that smoking trends differed among racial/ethnic populations and age groups. During 2000--2005, prenatal smoking rates increased among non-Hispanic white women aged 20--24 years and among non-Hispanic black women aged 25--34 years. However, these rates declined among other ages in these racial/ethnic populations. The increase in smoking rates among non-Hispanic white women aged 20--24 years is similar to the increase in smoking rates among young adults aged 18--24 years reported for 1991--2002 (26). This increase might be attributable to tobacco marketing directed at these age groups or to a cohort effect of teens who had high smoking rates in the 1990s. Further analyses are needed to explore the increases and disparities of prevalence rates among subpopulations of pregnant smokers.
Pregnancy is an opportune time to encourage women to quit smoking for their lifetime. The data provided in this report indicate that 53.0% of women who quit smoking during pregnancy reported smoking cigarettes at the time they completed the PRAMS questionnaire, an average of 4 months after delivery. In 2005, relapse rates varied among PRAMS sites (range: 36.4% [Oregon]--62.0% [Arkansas]). Women who were more likely to relapse to smoking after delivery were younger and had a lower annual income. Other important factors associated with relapse include living with a partner who smoked and experiencing a stressful life event and depression (27). Effective clinic-based interventions to prevent postpartum relapse do not exist; a meta-analysis of nine interventions to prevent relapse among pregnant and postpartum ex-smokers did not find any significant benefit (28). Relapse to smoking is common among quit attempts, and women should be encouraged to make additional attempts if relapse occurs. However, community-based interventions, (e.g., raising cigarette taxes) have been demonstrated to be effective in reducing relapse rates (11).
Limitations
Previous studies have determined that women underreport smoking and overreport quitting smoking (29,30). PRAMS is a self-administered, mailed, and confidential survey. More pregnant smokers are identified on the PRAMS questionnaire than the birth certificate, which is based on women reporting smoking to their health-care provider, suggesting that women might be more likely to report smoking through a confidential and anonymous survey than to their health-care providers (31,32). Underreporting of prenatal smoking was addressed by combining smoking reported on the PRAMS questionnaire and the linked birth certificate. However, because PRAMS data are based on self-reporting and are not validated biochemically, the data provided in this report probably underestimate the burden of smoking among pregnant women (33). In addition, PRAMS samples only women who have delivered a live infant and cannot estimate the total prevalence of smoking among all pregnant women, specifically those who did not have a live birth outcome (e.g., spontaneous abortions, ectopic pregnancies, or still-birth). Finally, the findings of this report are generalizable only to the PRAMS sites included in the analyses.
Conclusion
PRAMS is an effective state-level tool to monitor and evaluate the impact of tobacco-control activities. Additional resources for monitoring and evaluating tobacco-control efforts include 1) CDC's State Tobacco Activities Tracking and Evaluation system, which provides detailed information on state-level tobacco-control activities (e.g., tobacco excise taxes and smoke-free regulations in child care centers) and 2) CDC's Smoking-Attributable Mortality, Morbidity, and Economic Costs system, which estimates the health and health-related economic consequences of smoking to both adults and infants (34,35).
The majority of negative pregnancy outcomes are of unknown etiology (36). However, smoking during pregnancy is one behavior known to result in infant morbidity and mortality and for which effective cessation interventions exist. States can reduce smoking during pregnancy through sustained and comprehensive tobacco-control activities, including promoting policies that establish smoke-free environments in public places and the workplace, increasing tobacco excise taxes, banning all forms of tobacco advertisement, enforcing laws to prohibit sales to children and adolescents, using mass media campaigns, and ensuring adequate health-care coverage for cessation services.
On the clinical level, primary and prenatal health-care providers can assess their patients' smoking status, offer smoking cessation interventions, or refer smoking patients to effective cessation services. Maternal and child health-care practitioners should work in concert with state tobacco-control professionals to achieve the HP 2010 objective of reducing prenatal smoking to 1%.
Acknowledgments
The following persons assisted in the preparation of this report: E. Kathleen Adams, PhD, Rollins School of Public Health, Emory University, Atlanta, Georgia; Brian Morrow, MA, Dabo Brantley, MPH, Lucinda England, MD, Juliette Kendrick, MD, Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, CDC.
References
- Salihu HM, Aliyu MH, Pierre-Louis BJ, Alexander GR. Levels of excess infant deaths attributable to maternal smoking during pregnancy in the United States. Matern Child Health J 2003;7:219--27.
- CDC. Women and smoking: a report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, CDC; 2001.
- CDC. The health consequences of smoking: a report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, CDC; 2004.
- DiFranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children's health. Pediatrics 2004;113(Suppl 4):1007--15.
- Jedrychowski W, Flak E. Maternal smoking during pregnancy and postnatal exposure to environmental tobacco smoke as predisposition factors to acute respiratory infections. Environ Health Perspect 1997;105:302--6.
- CDC. The health consequences of involuntary exposure to tobacco smoke: a report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, CDC; 2006.
- CDC. Smoking-attributable mortality, years of potential life lost, and productivity losses---United States, 2000--2004. MMWR 2008;57:1226--8.
- Martin JA, Hamilton BE, Sutton PD, et al. Births: final data for 2005. Natl Vital Stat Rep 2007;56(6).
- CDC. Smoking prevalence among women of reproductive age---United States, 2006. MMWR 2008;57:849--52.
- US Department of Health and Human Services. Healthy people 2010. 2nd ed. With understanding and improving health and objectives for improving health (2 vols). Washington, DC: US Department of Health and Human Services; 2000.
- Colman GJ, Joyce T. Trends in smoking before, during, and after pregnancy in ten states. Am J Prev Med 2003;24:29--35.
- Suellentrop K, Morrow B, Williams L, D'Angelo D. Monitoring progress toward achieving maternal and infant Healthy People 2010 objectives---19 states, Pregnancy Risk Assessment Monitoring System (PRAMS), 2000--2003. In: Surveillance Summaries, October 6, 2006. MMWR 2006;55(No. SS-9).
- Shulman HB, Gilbert BC, Lansky A. The Pregnancy Risk Assessment Monitoring System (PRAMS): current methods and evaluation of 2001 response rates. Public Health Rep 2006;121:74--83.
- CDC. Pregnancy Risk Assessment Monitoring System (PRAMS): Questionnaire. Phase 4 (2000--2003) core questions, phase 4 (2000--2003) standard questions, phase 5 (2004--2008) core questions, and phase 5 (2004--2008) standard questions. Available at http://www.cdc.gov/PRAMS/Questionnaire.htm.
- Shah B, Barnwell B, Bieler G. SUDAAN user's manual: software for the statistical analysis of correlated data. Release 7.5. Research Triangle Park, NC: Research Triangle Institute; 1997.
- Hopkins DP, Briss PA, Ricard CJ, et al. Reviews of evidence regarding interventions to reduce tobacco use and exposure to environmental tobacco smoke. Am J Prev Med 2001;20(Suppl 2):16--66.
- CDC. State excise tax fact sheet. Atlanta, GA: US Department of Health and Human Services, CDC; 2007. Available at http://apps.nccd.cdc.gov/statesystem/publications/STATESystemFactSheetTax.pdf.
- Fiore MC, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, Gritz ER. Treating tobacco use and dependence: clinical practice guidelines. Rockville, MD, US Department of Health and Human Services, Public Health Service; 2008.
- Lumley J, Oliver SS, Chamberlain C, Oakley L. Interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev 2004;4:CD001055.
- Hartmann KE, Wechter ME, Payne P, Salisbury K, Jackson RD, Melvin CL. Best practice smoking cessation intervention and resource needs of prenatal care providers. Obstet Gynecol 2007;110:765--70.
- Price JH, Jordan TR, Dake JA. Obstetricians and gynecologists' perceptions and use of nicotine replacement therapy. J Community Health 2006;31:160--75.
- Tong VT, England LJ, Dietz PM, Asare LA. Smoking patterns and use of cessation interventions during pregnancy. Am J Prev Med 2008;
35:327--33. - US Department of Health and Human Services. 1-800-QUIT-NOW. Washington, DC: US Department of Health and Human Services; 2007. Available at http://1800quitnow.cancer.gov.
- CDC. State Medicaid coverage for tobacco-dependence treatments---United States, 2006. MMWR 2008;57:117--22.
- McMenamin SB, Halpin HA, Ibrahim JK, Orleans CT. Physician and enrollee knowledge of Medicaid coverage for tobacco dependence treatments. Am J Prev Med 2004;26:99--104.
- CDC. Cigarette smoking among adults---United States, 2004. MMWR 2005;54:1121--4.
- Fang WL, Goldstein AO, Butzen AY, et al. Smoking cessation in pregnancy: a review of postpartum relapse prevention strategies. J Am Board Fam Pract 2004;17:264--75.
- Hajek P, Stead LF, West R, Jarvis M. Relapse prevention interventions for smoking cessation. Cochrane Database Syst Rev 2005;1:CD003999.
- Ford RP, Tappin DM, Schluter PJ, Wild CJ. Smoking during pregnancy: how reliable are maternal self reports in New Zealand? J Epidemiol Community Health 1997;51:246--51.
- Lawrence T, Aveyard P, Croghan E. What happens to women's self-reported cigarette consumption and urinary cotinine levels in pregnancy? Addiction 2003;98:1315--20.
- Allen AM, Dietz PM, Tong VT, England L, Prince CB. Prenatal smoking prevalence ascertained from two population-based data sources: birth certificates and PRAMS questionnaires, 2004. Public Health Rep 2008;123:586--92.
- Northam S, Knapp TR. The reliability and validity of birth certificates. J Obstet Gynecol Neonatal Nurs 2006;35:3--12.
- Russell T, Crawford M, Woodby L. Measurements for active cigarette smoke exposure in prevalence and cessation studies: why simply asking pregnant women isn't enough. Nicotine Tob Res 2004(6 Suppl 2):
S141--51. - CDC. State Tobacco Activities Tracking and Evaluation (STATE) System. Atlanta, GA: US Department of Health and Human Services, CDC; 2008. Available at http://apps.nccd.cdc.gov/statesystem.
- CDC. Smoking-Attributable Mortality, Morbidity, and Economic Costs (SAMMEC). Atlanta, GA: US Department of health and Human Services, CDC; 2008. Available at http://apps.nccd.cdc.gov/sammec.
- Goldenberg RL, Rouse DJ. Prevention of premature birth. N Engl J Med 1998;339:313--20.