Appendices for U.S. Selected Practice Recommendations for Contraceptive Use, 2016
Appendix A
Summary Chart of U.S. Medical Eligibility Criteria for Contraceptive Use, 2016
Health-care providers can use the summary table as a quick reference guide to the classifications for hormonal contraceptive methods and intrauterine contraception to compare classifications across these methods ( Box A1) ( Table A1). For complete guidance, see the 2016 U.S. Medical Eligibility Criteria for Contraceptive Use (U.S. MEC) (Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep 2016;65[No. RR-3]) for clarifications to the numeric categories, as well as for summaries of the evidence and additional comments. Hormonal contraceptives and intrauterine devices do not protect against sexually transmitted diseases (STDs), including human immunodeficiency virus (HIV), and women using these methods should be counseled that consistent and correct use of the male latex condom reduces the risk for transmission of HIV and other STDs. Use of female condoms can provide protection from transmission of STDs, although data are limited.
 BOX A1. Categories for classifying hormonal contraceptives and intrauterine devices
BOX A1. Categories for classifying hormonal contraceptives and intrauterine devices
1 = A condition for which there is no restriction for the use of the contraceptive method.
2 = A condition for which the advantages of using the method generally outweigh the theoretical or proven risks.
3 = A condition for which the theoretical or proven risks usually outweigh the advantages of using the method.
4 = A condition that represents an unacceptable health risk if the contraceptive method is used.
![]() TABLE A1. Summary of classifications for hormonal contraceptive methods and intrauterine devices
TABLE A1. Summary of classifications for hormonal contraceptive methods and intrauterine devices
| Condition | Cu-IUD | LNG-IUD | Implants | DMPA | POP | CHCs | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Personal Characteristics and Reproductive History | ||||||||||||
| Pregnancy | 4* | 4* | NA* | NA * | NA * | NA * | ||||||
| Age | Menarche to <20 years: 2 | Menarche to <20 years: 2 | Menarche to <18 years: 1 | Menarche to <18 years: 2 | Menarche to <18 years: 1 | Menarche to <40 years: 1 | ||||||
| =20 years: 1 | =20 years: 1 | 18–45 years: 1 | 18–45 years: 1 | 18–45 years: 1 | =40 years: 2 | |||||||
| >45 years: 1 | >45 years: 2 | >45 years: 1 | ||||||||||
| Parity | ||||||||||||
| a. Nulliparous | 2 | 2 | 1 | 1 | 1 | 1 | ||||||
| b. Parous | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Breastfeeding | ||||||||||||
| a. <21 days postpartum | — | — | 2* | 2* | 2* | 4* | ||||||
| b. 21 to <30 days postpartum | ||||||||||||
| i. With other risk factors for VTE (e.g., age =35 years, previous VTE, thrombophilia, immobility, transfusion at delivery, peripartum cardiomyopathy, BMI =30 kg/m2, postpartum hemorrhage, postcesarean delivery, preeclampsia, or smoking) | — | — | 2* | 2* | 2* | 3* | ||||||
| ii. Without other risk factors for VTE | — | — | 2* | 2* | 2* | 3* | ||||||
| c. 30–42 days postpartum | ||||||||||||
| i. With other risk factors for VTE (e.g., age =35 years, previous VTE, thrombophilia, immobility, transfusion at delivery, peripartum cardiomyopathy, BMI =30 kg/m2, postpartum hemorrhage, postcesarean delivery, preeclampsia, or smoking) | — | — | 1* | 1* | 1* | 3* | ||||||
| ii. Without other risk factors for VTE | — | — | 1* | 1* | 1* | 2* | ||||||
| d. >42 days postpartum | — | — | 1* | 1* | 1* | 2* | ||||||
| Postpartum (nonbreastfeeding women) | ||||||||||||
| a. <21 days postpartum | — | — | 1 | 1 | 1 | 4 | ||||||
| b. 21–42 days postpartum | ||||||||||||
| i. With other risk factors for VTE (e.g., age =35 years, previous VTE, thrombophilia, immobility, transfusion at delivery, peripartum cardiomyopathy, BMI =30 kg/m2, postpartum hemorrhage, postcesarean delivery, preeclampsia, or smoking) | — | — | 1 | 1 | 1 | 3* | ||||||
| ii. Without other risk factors for VTE | — | — | 1 | 1 | 1 | 2 | ||||||
| c. >42 days postpartum | — | — | 1 | 1 | 1 | 1 | ||||||
| Postpartum (including cesarean delivery) | ||||||||||||
| a. <10 minutes after delivery of the placenta | ||||||||||||
| i. Breastfeeding | 1* | 2* | — | — | — | — | ||||||
| ii. Nonbreastfeeding | 1* | 1* | — | — | — | — | ||||||
| b. 10 minutes after delivery of the placenta to <4 weeks (breastfeeding or nonbreastfeeding) | 2* | 2* | — | — | — | — | ||||||
| c. =4 weeks (breastfeeding or nonbreastfeeding) | 1* | 1* | — | — | — | — | ||||||
| d. Postpartum sepsis | 4 | 4 | — | — | — | — | ||||||
| Postabortion | ||||||||||||
| a. First trimester | 1* | 1* | 1* | 1* | 1* | 1* | ||||||
| b. Second trimester | 2* | 2* | 1* | 1* | 1* | 1* | ||||||
| c. Immediate postseptic abortion | 4 | 4 | 1* | 1* | 1* | 1* | ||||||
| Past ectopic pregnancy | 1 | 1 | 1 | 1 | 2 | 1 | ||||||
| History of pelvic surgery (see Postpartum [Including Cesarean Delivery] section) | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Smoking | ||||||||||||
| a. Age <35 years | 1 | 1 | 1 | 1 | 1 | 2 | ||||||
| b. Age =35 years | ||||||||||||
| i. <15 cigarettes/day | 1 | 1 | 1 | 1 | 1 | 3 | ||||||
| ii. =15 cigarettes/day | 1 | 1 | 1 | 1 | 1 | 4 | ||||||
| Obesity | ||||||||||||
| a. BMI =30 kg/m2 | 1 | 1 | 1 | 1 | 1 | 2 | ||||||
| b. Menarche to <18 years and BMI =30 kg/m2 | 1 | 1 | 1 | 2 | 1 | 2 | ||||||
|
History of bariatric surgery This condition is associated with increased risk for adverse health events as a result of pregnancy. |
||||||||||||
| a. Restrictive procedures: decrease storage capacity of the stomach (vertical banded gastroplasty, laparoscopic adjustable gastric band, or laparoscopic sleeve gastrectomy) | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| b. Malabsorptive procedures: decrease absorption of nutrients and calories by shortening the functional length of the small intestine (Roux-en-Y gastric bypass or biliopancreatic diversion) | 1 | 1 | 1 | 1 | 3 | COCs: 3 | ||||||
| Patch and ring: 1 | ||||||||||||
| Cardiovascular Disease | ||||||||||||
| Multiple risk factors for atherosclerotic cardiovascular disease (e.g., older age, smoking, diabetes, hypertension, low HDL, high LDL, or high triglyceride levels) | 1 | 2 | 2* | 3* | 2* | 3/4* | ||||||
|
Hypertension Systolic blood pressure =160 mm Hg or diastolic blood pressure =100 mm Hg are associated with increased risk for adverse health events as a result of pregnancy. |
||||||||||||
| a. Adequately controlled hypertension | 1* | 1* | 1* | 2* | 1* | 3* | ||||||
| b. Elevated blood pressure levels (properly taken measurements) |
||||||||||||
| i. Systolic 140–159 mm Hg or diastolic 90–99 mm Hg | 1* | 1* | 1* | 2* | 1* | 3* | ||||||
| ii. Systolic =160 mm Hg or diastolic =100 mm Hg | 1* | 2* | 2* | 3* | 2* | 4* | ||||||
| c. Vascular disease | 1* | 2* | 2* | 3* | 2* | 4* | ||||||
| History of high blood pressure during pregnancy (when current blood pressure is measurable and normal) | 1 | 1 | 1 | 1 | 1 | 2 | ||||||
| Deep venous thrombosis/ Pulmonary embolism |
||||||||||||
| a. History of DVT/PE, not receiving anticoagulant therapy | ||||||||||||
| i. Higher risk for recurrent DVT/PE (one or more risk factors) | 1 | 2 | 2 | 2 | 2 | 4 | ||||||
| • History of estrogen-associated DVT/PE • Pregnancy-associated DVT/PE • Idiopathic DVT/PE • Known thrombophilia, including antiphospholipid syndrome • Active cancer (metastatic, receiving therapy, or within 6 months after clinical remission), excluding nonmelanoma skin cancer • History of recurrent DVT/PE |
||||||||||||
| ii. Lower risk for recurrent DVT/PE (no risk factors) | 1 | 2 | 2 | 2 | 2 | 3 | ||||||
| b. Acute DVT/PE | 2 | 2 | 2 | 2 | 2 | 4 | ||||||
| c. DVT/PE and established receiving anticoagulant therapy for at least 3 months | ||||||||||||
| i. Higher risk for recurrent DVT/PE (one or more risk factors) | 2 | 2 | 2 | 2 | 2 | 4* | ||||||
| • Known thrombophilia, including antiphospholipid syndrome • Active cancer (metastatic, receiving therapy, or within 6 months after clinical remission), excluding nonmelanoma skin cancer • History of recurrent DVT/PE |
||||||||||||
| ii. Lower risk for recurrent DVT/PE (no risk factors) | 2 | 2 | 2 | 2 | 2 | 3* | ||||||
| d. Family history (first-degree relatives) | 1 | 1 | 1 | 1 | 1 | 2 | ||||||
| e. Major surgery | ||||||||||||
| i. With prolonged immobilization | 1 | 2 | 2 | 2 | 2 | 4 | ||||||
| ii. Without prolonged immobilization | 1 | 1 | 1 | 1 | 1 | 2 | ||||||
| f. Minor surgery without immobilization | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
|
Known thrombogenic mutations (e.g., factor V Leiden; prothrombin mutation; and protein S, protein C, and antithrombin deficiencies) This condition is associated with increased risk for adverse health events as a result of pregnancy. |
1* | 2* | 2* | 2* | 2* | 4* | ||||||
| Superficial venous disorders | ||||||||||||
| a. Varicose veins | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| b. Superficial venous thrombosis (acute or history) | 1 | 1 | 1 | 1 | 1 | 3* | ||||||
|
Current and history of ischemic heart disease This condition is associated with increased risk for adverse health events as a result of pregnancy. |
Initiation | Continuation | Initiation | Continuation | Initiation | Continuation | ||||||
| 1 | 2 | 3 | 2 | 3 | 3 | 2 | 3 | 4 | ||||
|
Stroke (history of cerebrovascular accident) This condition is associated with increased risk for adverse health events as a result of pregnancy. |
Initiation | Continuation | Initiation | Continuation | ||||||||
| 1 | 2 | 2 | 3 | 3 | 2 | 3 | 4 | |||||
|
Valvular heart disease Complicated valvular heart disease is associated with increased risk for adverse health events as a result of pregnancy. |
||||||||||||
| a. Uncomplicated | 1 | 1 | 1 | 1 | 1 | 2 | ||||||
| b. Complicated (pulmonary hypertension, risk for atrial fibrillation, or history of subacute bacterial endocarditis) | 1 | 1 | 1 | 1 | 1 | 4 | ||||||
|
Peripartum cardiomyopathy This condition is associated with increased risk for adverse health events as a result of pregnancy. |
||||||||||||
| a. Normal or mildly impaired cardiac function (New York Heart Association Functional Class I or II: patients with no limitation of activities or patients with slight, mild limitation of activity) (2) | ||||||||||||
| i. <6 months | 2 | 2 | 1 | 1 | 1 | 4 | ||||||
| ii. =6 months | 2 | 2 | 1 | 1 | 1 | 3 | ||||||
| b. Moderately or severely impaired cardiac function (New York Heart Association Functional Class III or IV: patients with marked limitation of activity or patients who should be at complete rest) (2). | 2 | 2 | 2 | 2 | 2 | 4 | ||||||
| Rheumatic Diseases | ||||||||||||
|
Systemic lupus erythematosus This condition is associated with increased risk for adverse health events as a result of pregnancy. |
Initiation | Continuation | Initiation | Continuation | ||||||||
| a. Positive (or unknown) antiphospholipid antibodies | 1* | 1* | 3* | 3* | 3* | 3* | 3* | 4* | ||||
| b. Severe thrombocytopenia | 3* | 2* | 2* | 2* | 3* | 2* | 2* | 2* | ||||
| c. Immunosuppressive therapy | 2* | 1* | 2* | 2* | 2* | 2* | 2* | 2* | ||||
| d. None of the above | 1* | 1* | 2* | 2* | 2* | 2* | 2* | 2* | ||||
| Rheumatoid arthritis | Initiation | Continuation | Initiation | Continuation | ||||||||
| a. Receiving immunosuppressive therapy | 2 | 1 | 2 | 1 | 1 | 2/3* | 1 | 2 | ||||
| b. Not receiving immunosuppressive therapy | 1 | 1 | 1 | 2 | 1 | 2 | ||||||
| Neurologic Conditions | ||||||||||||
| Headaches | ||||||||||||
| a. Nonmigraine (mild or severe) | 1 | 1 | 1 | 1 | 1 | 1* | ||||||
| b. Migraine | ||||||||||||
| i. Without aura (This category of migraine includes menstrual migraine.) | 1 | 1 | 1 | 1 | 1 | 2* | ||||||
| ii. With aura | 1 | 1 | 1 | 1 | 1 | 4* | ||||||
|
Epilepsy This condition is associated with increased risk for adverse health events as a result of pregnancy. |
1 | 1 | 1* | 1* | 1* | 1* | ||||||
| Multiple sclerosis | ||||||||||||
| a. With prolonged immobility | 1 | 1 | 1 | 2 | 1 | 3 | ||||||
| b. Without prolonged immobility | 1 | 1 | 1 | 2 | 1 | 1 | ||||||
| Depressive Disorders | ||||||||||||
| Depressive disorders | 1* | 1* | 1* | 1* | 1* | 1* | ||||||
| Reproductive Tract Infections and Disorders | ||||||||||||
| Vaginal bleeding patterns | Initiation | Continuation | ||||||||||
| a. Irregular pattern without heavy bleeding | 1 | 1 | 1 | 2 | 2 | 2 | 1 | |||||
| b. Heavy or prolonged bleeding (includes regular and irregular patterns) | 2* | 1* | 2* | 2* | 2* | 2* | 1* | |||||
|
Unexplained vaginal bleeding (suspicious for serious condition) before evaluation |
Initiation | Continuation | Initiation | Continuation | ||||||||
| 4* | 2* | 4* | 2* | 3* | 3* | 2* | 2* | |||||
| Endometriosis | 2 | 1 | 1 | 1 | 1 | 1 | ||||||
| Benign ovarian tumors (including cysts) | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Severe dysmenorrhea | 2 | 1 | 1 | 1 | 1 | 1 | ||||||
|
Gestational trophoblastic disease This condition is associated with increased risk for adverse health events as a result of pregnancy. |
||||||||||||
| a. Suspected gestational trophoblastic disease (immediate postevacuation) | ||||||||||||
| i. Uterine size first trimester | 1* | 1* | 1* | 1* | 1* | 1* | ||||||
| ii. Uterine size second trimester | 2* | 2* | 1* | 1* | 1* | 1* | ||||||
| b. Confirmed gestational trophoblastic disease (after initial evacuation and during monitoring) | Initiation | Continuation | Initiation | Continuation | ||||||||
| i. Undetectable/nonpregnant ß-hCG levels | 1* | 1* | 1* | 1* | 1* | 1* | 1* | 1* | ||||
| ii. Decreasing ß-hCG levels | 2* | 1* | 2* | 1* | 1* | 1* | 1* | 1* | ||||
| iii. Persistently elevated ß-hCG levels or malignant disease, with no evidence or suspicion of intrauterine disease | 2* | 1* | 2* | 1* | 1* | 1* | 1* | 1* | ||||
| iv. Persistently elevated ß-hCG levels or malignant disease, with evidence or suspicion of intrauterine disease | 4* | 2* | 4* | 2* | 1* | 1* | 1* | 1* | ||||
| Cervical ectropion | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Cervical intraepithelial neoplasia | 1 | 2 | 2 | 2 | 1 | 2 | ||||||
| Cervical cancer (awaiting treatment) | Initiation | Continuation | Initiation | Continuation | ||||||||
| 4 | 2 | 4 | 2 | 2 | 2 | 1 | 2 | |||||
|
Breast disease Breast cancer is associated with increased risk of adverse health events as a result of pregnancy. |
||||||||||||
| a. Undiagnosed mass | 1 | 2 | 2* | 2* | 2* | 2* | ||||||
| b. Benign breast disease | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| c. Family history of cancer | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| d. Breast cancer | ||||||||||||
| i. Current | 1 | 4 | 4 | 4 | 4 | 4 | ||||||
| ii. Past and no evidence of current disease for 5 years | 1 | 3 | 3 | 3 | 3 | 3 | ||||||
| Endometrial hyperplasia | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
|
Endometrial cancer This condition is associated with increased risk for adverse health events as a result of pregnancy. |
Initiation | Continuation | Initiation | Continuation | ||||||||
| 4 | 2 | 4 | 2 | 1 | 1 | 1 | 1 | |||||
|
Ovarian cancer This condition is associated with increased risk for adverse health events as a result of pregnancy. |
1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Uterine fibroids | 2 | 2 | 1 | 1 | 1 | 1 | ||||||
| Anatomical abnormalities | ||||||||||||
| a. Distorted uterine cavity (any congenital or acquired uterine abnormality distorting the uterine cavity in a manner that is incompatible with IUD insertion) | 4 | 4 | — | — | — | — | ||||||
| b. Other abnormalities (including cervical stenosis or cervical lacerations) not distorting the uterine cavity or interfering with IUD insertion | 2 | 2 | — | — | — | — | ||||||
| Pelvic inflammatory disease | ||||||||||||
| a. Past PID (assuming no current risk factors for STDs) | Initiation | Continuation | Initiation | Continuation | ||||||||
| i. With subsequent pregnancy | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| ii. Without subsequent pregnancy | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | ||||
| b. Current PID | 4 | 2* | 4 | 2* | 1 | 1 | 1 | 1 | ||||
| Sexually transmitted diseases | Initiation | Continuation | Initiation | Continuation | ||||||||
| a. Current purulent cervicitis or chlamydial infection or gonococcal infection | 4 | 2* | 4 | 2* | 1 | 1 | 1 | 1 | ||||
| b. Vaginitis (including Trichomonas vaginalis and bacterial vaginosis) | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | ||||
| c. Other factors related to STDs | 2* | 2 | 2* | 2 | 1 | 1 | 1 | 1 | ||||
| HIV | ||||||||||||
| Initiation | Continuation | Initiation | Continuation | |||||||||
| High risk for HIV | 2 | 2 | 2 | 2 | 1 | 1* | 1 | 1 | ||||
|
HIV infection For women with HIV infection who are not clinically well or not receiving ARV therapy, this condition is associated with increased risk for adverse health events as a result of pregnancy. |
— | — | — | — | 1* | 1* | 1* | 1* | ||||
| a. Clinically well receiving ARV therapy | 1 | 1 | 1 | 1 | — | — | — | — | ||||
| b. Not clinically well or not receiving ARV therapy | 2 | 1 | 2 | 1 | — | — | — | — | ||||
| Other Infections | ||||||||||||
|
Schistosomiasis Schistosomiasis with fibrosis of the liver is associated with increased risk for adverse health events as a result of pregnancy. |
||||||||||||
| a. Uncomplicated | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| b. Fibrosis of the liver (if severe, see Cirrhosis) | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
|
Tuberculosis This condition is associated with increased risk for adverse health events as a result of pregnancy. |
Initiation | Continuation | Initiation | Continuation | ||||||||
| a. Nonpelvic | 1 | 1 | 1 | 1 | 1* | 1* | 1* | 1* | ||||
| b. Pelvic | 4 | 3 | 4 | 3 | 1* | 1* | 1* | 1* | ||||
| Malaria | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Endocrine Conditions | ||||||||||||
|
Diabetes Insulin-dependent diabetes; diabetes with nephropathy, retinopathy, neuropathy, or diabetes with other vascular disease; or diabetes of >20 years’ duration are associated with increased risk of adverse health events as a result of pregnancy. |
||||||||||||
| a. History of gestational disease | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| b. Nonvascular disease | ||||||||||||
| i. Non-insulin dependent | 1 | 2 | 2 | 2 | 2 | 2 | ||||||
| ii. Insulin dependent | 1 | 2 | 2 | 2 | 2 | 2 | ||||||
| c. Nephropathy, retinopathy, or neuropathy | 1 | 2 | 2 | 3 | 2 | 3/4* | ||||||
| d. Other vascular disease or diabetes of >20 years’ duration | 1 | 2 | 2 | 3 | 2 | 3/4* | ||||||
| Thyroid disorders | ||||||||||||
| a. Simple goiter | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| b. Hyperthyroid | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| c. Hypothyroid | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Gastrointestinal Conditions | ||||||||||||
| Inflammatory bowel disease (ulcerative colitis or Crohn’s disease) | 1 | 1 | 1 | 2 | 2 | 2/3* | ||||||
| Gallbladder disease | ||||||||||||
| a. Symptomatic | ||||||||||||
| i. Treated by cholecystectomy | 1 | 2 | 2 | 2 | 2 | 2 | ||||||
| ii. Medically treated | 1 | 2 | 2 | 2 | 2 | 3 | ||||||
| iii. Current | 1 | 2 | 2 | 2 | 2 | 3 | ||||||
| b. Asymptomatic | 1 | 2 | 2 | 2 | 2 | 2 | ||||||
| History of cholestasis | ||||||||||||
| a. Pregnancy related | 1 | 1 | 1 | 1 | 1 | 2 | ||||||
| b. Past COC related | 1 | 2 | 2 | 2 | 2 | 3 | ||||||
| Viral hepatitis | Initiation | Continuation | ||||||||||
| a. Acute or flare | 1 | 1 | 1 | 1 | 1 | 3/4* | 2 | |||||
| b. Carrier | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||
| c. Chronic | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||
|
Cirrhosis Severe cirrhosis is associated with increased risk for adverse health events as a result of pregnancy. |
||||||||||||
| a. Mild (compensated) | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| b. Severe (decompensated) | 1 | 3 | 3 | 3 | 3 | 4 | ||||||
|
Liver tumors Hepatocellular adenoma and malignant liver tumors are associated with increased risk for adverse health events as a result of pregnancy. |
||||||||||||
| a. Benign | ||||||||||||
| i. Focal nodular hyperplasia | 1 | 2 | 2 | 2 | 2 | 2 | ||||||
| ii. Hepatocellular adenoma | 1 | 3 | 3 | 3 | 3 | 4 | ||||||
| b. Malignant (hepatoma) | 1 | 3 | 3 | 3 | 3 | 4 | ||||||
| Respiratory Conditions | ||||||||||||
|
Cystic fibrosis This condition is associated with increased risk for adverse health events as a result of pregnancy. |
1* | 1* | 1* | 2* | 1* | 1* | ||||||
| Anemias | ||||||||||||
| Thalassemia | 2 | 1 | 1 | 1 | 1 | 1 | ||||||
|
Sickle cell disease This condition is associated with increased risk for adverse health events as a result of pregnancy. |
2 | 1 | 1 | 1 | 1 | 2 | ||||||
| Iron-deficiency anemia | 2 | 1 | 1 | 1 | 1 | 1 | ||||||
| Solid Organ Transplantation | ||||||||||||
|
Solid organ transplantation This condition is associated with increased risk for adverse health events as a result of pregnancy. |
Initiation | Continuation | Initiation | Continuation | ||||||||
| a. Complicated: graft failure (acute or chronic), rejection, or cardiac allograft vasculopathy | 3 | 2 | 3 | 2 | 2 | 2 | 2 | 4 | ||||
| b. Uncomplicated | 2 | 2 | 2 | 2 | 2 | 2* | ||||||
| Drug Interactions | ||||||||||||
| Antiretroviral therapy | Initiation | Continuation | Initiation | Continuation | ||||||||
| a. Nucleoside reverse transcriptase inhibitors (NRTIs) | ||||||||||||
| i. Abacavir (ABC) | 1/2* | 1* | 1/2* | 1* | 1 | 1 | 1 | 1 | ||||
| ii. Tenofovir (TDF) | 1/2* | 1* | 1/2* | 1* | 1 | 1 | 1 | 1 | ||||
| iii. Zidovudine (AZT) | 1/2* | 1* | 1/2* | 1* | 1 | 1 | 1 | 1 | ||||
| iv. Lamivudine (3TC) | 1/2* | 1* | 1/2* | 1* | 1 | 1 | 1 | 1 | ||||
| v. Didanosine (DDI) | 1/2* | 1* | 1/2* | 1* | 1 | 1 | 1 | 1 | ||||
| vi. Emtricitabine (FTC) | 1/2* | 1* | 1/2* | 1* | 1 | 1 | 1 | 1 | ||||
| vii. Stavudine (D4T) | 1/2* | 1* | 1/2* | 1* | 1 | 1 | 1 | 1 | ||||
| b. Nonnucleoside reverse transcriptase inhibitors (NNRTIs) | ||||||||||||
| i. Efavirenz (EFV) | 1/2* | 1* | 1/2* | 1* | 2* | 1* | 2* | 2* | ||||
| ii. Etravirine (ETR) | 1/2* | 1* | 1/2* | 1* | 1 | 1 | 1 | 1 | ||||
| iii. Nevirapine (NVP) | 1/2* | 1* | 1/2* | 1* | 1 | 1 | 1 | 1 | ||||
| iv. Rilpivirine (RPV) | 1/2* | 1* | 1/2* | 1* | 1 | 1 | 1 | 1 | ||||
| c. Ritonavir-boosted protease inhibitors | ||||||||||||
| i. Ritonavir-boosted atazanavir (ATV/r) | 1/2* | 1* | 1/2* | 1* | 2* | 1* | 2* | 2* | ||||
| ii. Ritonavir-boosted darunavir (DRV/r) | 1/2* | 1* | 1/2* | 1* | 2* | 1* | 2* | 2* | ||||
| iii. Ritonavir-boosted fosemprenavir (FPV/r) | 1/2* | 1* | 1/2* | 1* | 2* | 1* | 2* | 2* | ||||
| iv. Ritonavir-boosted lopinavir (LPV/r) | 1/2* | 1* | 1/2* | 1* | 1 | 1 | 1 | 1 | ||||
| v. Ritonavir-boosted saquinavir (SQV/r) | 1/2* | 1* | 1/2* | 1* | 2* | 1* | 2* | 2* | ||||
| vi. Ritonavir-boosted tipranavir (TPV/r) | 1/2* | 1* | 1/2* | 1* | 2* | 1* | 2* | 2* | ||||
| d. Protease inhibitors without ritonavir | ||||||||||||
| i. Atazanavir (ATV) | 1/2* | 1* | 1/2* | 1* | 1 | 1 | 1 | 2* | ||||
| ii. Fosamprenavir (FPV) | 1/2* | 1* | 1/2* | 1* | 2* | 2* | 2* | 3* | ||||
| iii. Indinavir (IDV) | 1/2* | 1* | 1/2* | 1* | 1 | 1 | 1 | 1 | ||||
| iv. Nelfinavir (NFV) | 1/2* | 1* | 1/2* | 1* | 2* | 1* | 2* | 2* | ||||
| e. CCR5 co-receptor antagonists | ||||||||||||
| i. Maraviroc (MVC) | 1/2* | 1* | 1/2* | 1* | 1 | 1 | 1 | 1 | ||||
| f. HIV integrase strand transfer inhibitors | ||||||||||||
| i. Raltegravir (RAL) | 1/2* | 1* | 1/2* | 1* | 1 | 1 | 1 | 1 | ||||
| ii. Dolutegravir (DTG) | 1/2* | 1* | 1/2* | 1* | 1 | 1 | 1 | 1 | ||||
| iii. Elvitegravir (EVG) | 1/2* | 1* | 1/2* | 1* | 1 | 1 | 1 | 1 | ||||
| g. Fusion inhibitors | ||||||||||||
| i. Enfuvirtide | 1/2* | 1* | 1/2* | 1* | 1 | 1 | 1 | 1 | ||||
| Anticonvulsant therapy | ||||||||||||
| a. Certain anticonvulsants (phenytoin, carbamazepine, barbiturates, primidone, topiramate, and oxcarbazepine) | 1 | 1 | 2* | 1* | 3* | 3* | ||||||
| b. Lamotrigine | 1 | 1 | 1 | 1 | 1 | 3* | ||||||
| Antimicrobial therapy | ||||||||||||
| a. Broad-spectrum antibiotics | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| b. Antifungals | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| c. Antiparasitics | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| d. Rifampin or rifabutin therapy | 1 | 1 | 2* | 1* | 3* | 3* | ||||||
| Psychotropic medications | ||||||||||||
| a. SSRIs | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| St. John’s wort | 1 | 1 | 2 | 1 | 2 | 2 | ||||||
Abbreviations: BMI = body mass index; COC = combined oral contraceptive; Cu-IUD = copper-containing IUD; DMPA = depot medroxyprogesterone acetate; DVT = deep venous thrombosis; hCG = human chorionic gonadotropin; HDL = high-density lipoprotein; HIV = human immunodeficiency virus.; IUD = intrauterine device; LDL = low-density lipoprotein; LNG-IUD = levonorgestrel-releasing IUD; NA = not applicable; PE = pulmonary embolism; PID = pelvic inflammatory disease; POP = progestin-only pill; SSRI = selective serotonin reuptake inhibitor; STD = sexually transmitted disease.
*Consult the respective appendix for each contraceptive method in the 2016 U.S. Medical Eligibility Criteria for Contraceptive Use (1) for clarifications to the numeric categories.
References
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep 2016;65(No. RR-3).
- The Criteria Committee of the New York Heart Association. Nomenclature and criteria for diagnosis of diseases of the heart and great vessels. 9th ed. Boston, MA: Little, Brown & Co; 1994.
Appendix B
When To Start Using Specific Contraceptive Methods
| Contraceptive method | When to start (if the provider is reasonably certain that the woman is not pregnant) | Additional contraception (i.e., back-up) needed | Examinations or tests needed before initiation* |
|---|---|---|---|
| Copper-containing IUD | Anytime | Not needed | Bimanual examination and cervical inspection† |
| Levonorgestrel-releasing IUD | Anytime | If >7 days after menses started, use back-up method or abstain for 7 days. | Bimanual examination and cervical inspection† |
| Implant | Anytime | If >5 days after menses started, use back-up method or abstain for 7 days. | None |
| Injectable | Anytime | If >7 days after menses started, use back-up method or abstain for 7 days. | None |
| Combined hormonal contraceptive | Anytime | If >5 days after menses started, use back-up method or abstain for 7 days. | Blood pressure measurement |
| Progestin-only pill | Anytime | If >5 days after menses started, use back-up method or abstain for 2 days. | None |
Abbreviations: BMI = body mass index; HIV = human immunodeficiency virus; IUD = intrauterine device; STD = sexually transmitted disease; U.S. MEC = U.S. Medical Eligibility Criteria for Contraceptive Use.
* Weight (BMI) measurement is not needed to determine medical eligibility for any methods of contraception because all methods can be used (U.S. MEC 1) or generally can be used (U.S. MEC 2) among obese women (Box 1). However, measuring weight and calculating BMI (weight [kg] / height [m]2) at baseline might be helpful for monitoring any changes and counseling women who might be concerned about weight change perceived to be associated with their contraceptive method.
† Most women do not require additional STD screening at the time of IUD insertion. If a woman with risk factors for STDs has not been screened for gonorrhea and chlamydia according to CDC’s STD Treatment Guidelines (http://www.cdc.gov/std/treatment), screening can be performed at the time of IUD insertion, and insertion should not be delayed. Women with current purulent cervicitis or chlamydial infection or gonococcal infection should not undergo IUD insertion (U.S. MEC 4).
Appendix C
Examinations and Tests Needed Before Initiation of Contraceptive Methods
The examinations or tests noted apply to women who are presumed to be healthy ( Table C1). Those with known medical problems or other special conditions might need additional examinations or tests before being determined to be appropriate candidates for a particular method of contraception. The 2016 U.S. Medical Eligibility Criteria for Contraceptive Use (U.S. MEC) might be useful in such circumstances (1). The following classification was considered useful in differentiating the applicability of the various examinations or tests:
-
Class A: essential and mandatory in all circumstances for safe and effective use of the contraceptive method.
-
Class B: contributes substantially to safe and effective use, but implementation may be considered within the public health and/or service context; risk of not performing an examination or test should be balanced against the benefits of making the contraceptive method available.
-
Class C: does not contribute substantially to safe and effective use of the contraceptive method.
These classifications focus on the relationship of the examinations or tests to safe initiation of a contraceptive method. They are not intended to address the appropriateness of these examinations or tests in other circumstances. For example, some of the examinations or tests that are not deemed necessary for safe and effective contraceptive use might be appropriate for good preventive health care or for diagnosing or assessing suspected medical conditions. Any additional screening needed for preventive health care can be performed at the time of contraception initiation and initiation should not be delayed for test results.
No examinations or tests are needed before initiating condoms or spermicides. A bimanual examination is necessary for diaphragm fitting. A bimanual examination and cervical inspection are needed for cervical cap fitting.
References
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep 2016;65(No. RR-3).
![]() TABLE C1. Examinations and tests needed before initiation of contraceptive methods
TABLE C1. Examinations and tests needed before initiation of contraceptive methods
| Examination or test | Contraceptive method and class | |||||||
|---|---|---|---|---|---|---|---|---|
| Cu-IUD and LNG-IUD | Implant | Injectable | CHC | POP | Condom | Diaphragm or cervical cap |
Spermicide | |
| Examination | ||||||||
| Blood pressure | C | C | C | A* | C | C | C | C |
| Weight (BMI) (weight [kg] / height [m]2) | —† | —† | —† | —† | —† | C | C | C |
| Clinical breast examination | C | C | C | C | C | C | C | C |
| Bimanual examination and cervical inspection | A | C | C | C | C | C | A§ | C |
| Laboratory test | ||||||||
| Glucose | C | C | C | C | C | C | C | C |
| Lipids | C | C | C | C | C | C | C | C |
| Liver enzymes | C | C | C | C | C | C | C | C |
| Hemoglobin | C | C | C | C | C | C | C | C |
| Thrombogenic mutations | C | C | C | C | C | C | C | C |
| Cervical cytology (Papanicolaou test) | C | C | C | C | C | C | C | C |
| STD screening with laboratory tests | —¶ | C | C | C | C | C | C | C |
| HIV screening with laboratory tests | C | C | C | C | C | C | C | C |
Abbreviations: BMI = body mass index; CHC = combined hormonal contraceptive; Cu-IUD = copper-containing intrauterine device; HIV = human immunodeficiency virus; LNG-IUD = levonorgestrel-releasing intrauterine device; POP = progestin-only pill; STD = sexually transmitted disease; U.S. MEC = U.S. Medical Eligibility Criteria for Contraceptive Use.
* In instances in which blood pressure cannot be measured by a provider, blood pressure measured in other settings can be reported by the woman to her provider.
† Weight (BMI) measurement is not needed to determine medical eligibility for any methods of contraception because all methods can be used (U.S. MEC 1) or generally can be used (U.S. MEC 2) among obese women (Box 1). However, measuring weight and calculating BMI at baseline might be helpful for monitoring any changes and counseling women who might be concerned about weight change perceived to be associated with their contraceptive method.
§A bimanual examination (not cervical inspection) is needed for diaphragm fitting.
¶ Most women do not require additional STD screening at the time of IUD insertion. If a woman with risk factors for STDs has not been screened for gonorrhea and chlamydia according to CDC’s STD Treatment Guidelines (http://www.cdc.gov/std/treatment), screening can be performed at the time of IUD insertion, and insertion should not be delayed. Women with current purulent cervicitis or chlamydial infection or gonococcal infection should not undergo IUD insertion (U.S. MEC 4).
Appendix D
Routine Follow-Up After Contraceptive Initiation
These recommendations address when routine follow-up is recommended for safe and effective continued use of contraception for healthy women ( Table D1). The recommendations refer to general situations and might vary for different users and different situations. Specific populations who might benefit from frequent follow-up visits include adolescents, those with certain medical conditions or characteristics, and those with multiple medical conditions.
TABLE D1. Routine follow-up after contraceptive initiation
| Action | Contraceptive method | ||||
|---|---|---|---|---|---|
| Cu-IUD or LNG-IUD | Implant | Injectable | CHC | POP | |
| General follow-up | |||||
| Advise women to return at any time to discuss side effects or other problems or if they want to change the method. Advise women using IUDs, implants, or injectables when the IUD or implant needs to be removed or when a reinjection is needed. No routine follow-up visit is required. | X | X | X | X | X |
| Other routine visits | |||||
| Assess the woman’s satisfaction with her current method and whether she has any concerns about method use. | X | X | X | X | X |
| Assess any changes in health status, including medications, that would change the method’s appropriateness for safe and effective continued use based on U.S. MEC (i.e., category 3 and 4 conditions and characteristics) (Box 1). | X | X | X | X | X |
| Consider performing an examination to check for the presence of IUD strings. | X | — | — | — | — |
| Consider assessing weight changes and counseling women who are concerned about weight change perceived to be associated with their contraceptive method. | X | X | X | X | X |
| Measure blood pressure. | — | — | — | X | — |
Abbreviations: CHC = combined hormonal contraceptives; Cu-IUD = copper-containing intrauterine device; HIV = human immunodeficiency virus; IUD = intrauterine device; LNG-IUD = levonorgestrel-releasing intrauterine device; POP = progestin-only pills; U.S. MEC = U.S. Medical Eligibility Criteria for Contraceptive Use.
Appendix E
Management of Women with Bleeding Irregularities While Using Contraception*
Management of women with bleeding irregularities while using contraception
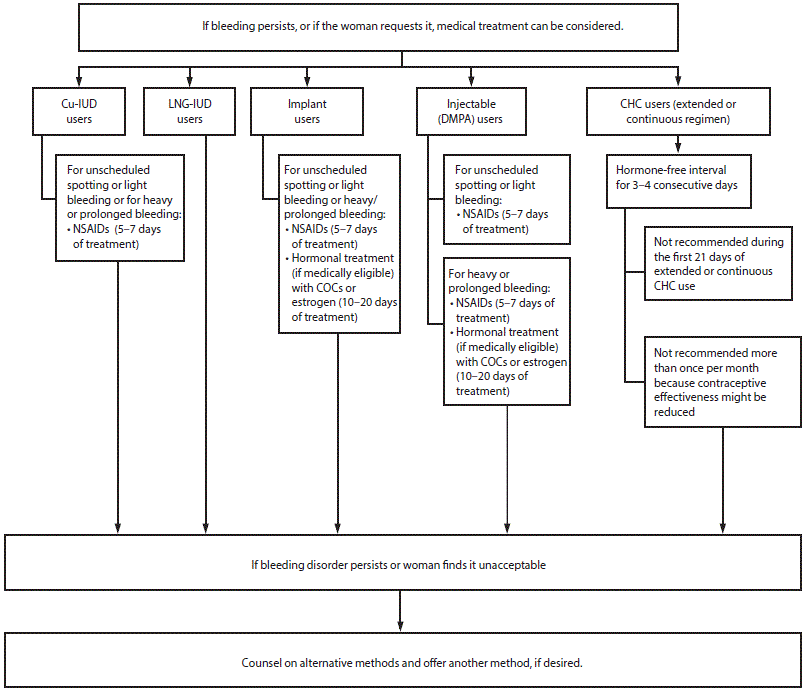
Abbreviations: CHC = combined hormonal contraceptive; COC = combined oral contraceptive; Cu-IUD = copper-containing intrauterine device; DMPA = depot medroxyprogesterone acetate; LNG-IUD = levonorgestrel-releasing intrauterine device; NSAIDs = nonsteroidal antiinflammatory drugs.
* If clinically warranted, evaluate for underlying condition. Treat the condition or refer for care. Heavy or prolonged bleeding, either unscheduled or menstrual, is uncommon among LNG-IUD users and implant users.
Appendix F
Management of Intrauterine Devices When Users are Found To Have Pelvic Inflammatory Disease
Management of intrauterine devices when users of copper-containing intrauterine devices or levonorgestrel-releasing intrauterine devices are found to have pelvic inflammatory disease
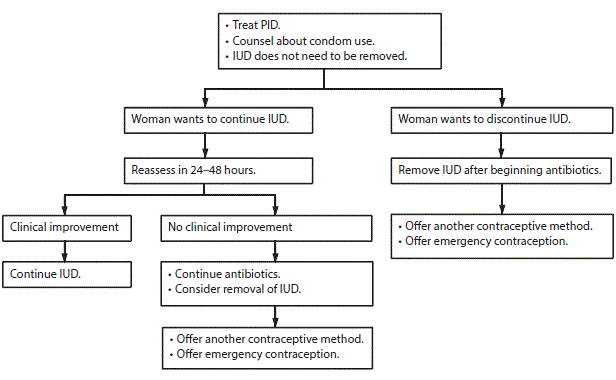
Abbreviations: IUD = intrauterine device; PID = pelvic inflammatory disease.
* Treat according to the CDC Sexually Transmitted Diseases Treatment Guidelines (http://www.cdc.gov/std/treatment).
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (https://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.
- Page last reviewed: July 28, 2016
- Page last updated: July 28, 2016
- Content source:


 ShareCompartir
ShareCompartir