Malaria Surveillance — United States, 2013
Surveillance Summaries / March 4, 2016 / 65(2);1–22
Karen A. Cullen, PhD; Kimberly E. Mace, PhD; Paul M. Arguin, MD
Author Affiliation: Malaria Branch, Division of Parasitic Diseases and Malaria, Center for Global Health, CDC
Abstract
Problem/Condition: Malaria in humans is caused by intraerythrocytic protozoa of the genus Plasmodium. These parasites are transmitted by the bite of an infective female Anopheles mosquito. The majority of malaria infections in the United States occur among persons who have traveled to regions with ongoing malaria transmission. However, malaria is also occasionally acquired by persons who have not traveled out of the country through exposure to infected blood products, congenital transmission, laboratory exposure, or local mosquitoborne transmission. Malaria surveillance in the United States is conducted to identify episodes of local transmission and to guide prevention recommendations for travelers.
Period Covered: This report summarizes cases in persons with onset of illness in 2013 and summarizes trends during previous years.
Description of System: Malaria cases diagnosed by blood film, polymerase chain reaction, or rapid diagnostic tests are mandated to be reported to local and state health departments by health care providers or laboratory staff. Case investigations are conducted by local and state health departments, and reports are transmitted to CDC through the National Malaria Surveillance System, National Notifiable Diseases Surveillance System, or direct CDC consultations. CDC conducted antimalarial drug resistance marker testing on blood samples submitted to CDC by health care providers or local/state health departments. Data from these reporting systems serve as the basis for this report.
Results: CDC received 1,727 reported cases of malaria, including two congenital cases, with an onset of symptoms in 2013 among persons in the United States. The total number of cases represents a 2% increase from the 1,687 cases reported for 2012. Plasmodium falciparum, P. vivax, P. malariae, and P. ovale were identified in 61%, 14%, 3%, and 4% of cases, respectively. Forty (2%) patients were infected by two species. The infecting species was unreported or undetermined in 17% of cases. Polymerase chain reaction testing determined or corrected the species for 85 of the 137 (62%) samples evaluated for drug resistance marker testing. Of the 904 patients who reported purpose of travel, 635 (70%) were visiting friends or relatives (VFR). Among the 961 cases in U.S. civilians for whom information on chemoprophylaxis use and travel region was known, 42 (4%) patients reported that they had initiated and adhered to a chemoprophylaxis drug regimen recommended by CDC for the regions to which they had traveled. Thirty-six cases were reported in pregnant women, none of whom had adhered to chemoprophylaxis. Among all reported cases, approximately 270 (16%) were classified as severe illnesses in 2013. Of these, 10 persons with malaria died in 2013, the highest number since 2001. In 2013, a total of 137 blood samples submitted to CDC were tested for molecular markers associated with antimalarial drug resistance. Of the 100 P. falciparum-positive samples, 95 were tested for pyrimethamine resistance: 88 (93%) had genetic polymorphisms associated with pyrimethamine drug resistance, 74 (76%) with sulfadoxine resistance, 53 (53%) with chloroquine resistance, one (1%) with atovaquone resistance, none with mefloquine drug resistance, and none with artemisinin resistance.
Interpretation: The overall trend of malaria cases has been increasing since 1973; the number of cases reported in 2013 is the third highest annual total since then. Despite progress in reducing the global burden of malaria, the disease remains endemic in many regions, and the use of appropriate prevention measures by travelers is still inadequate.
Public Health Actions: Completion of data elements on the malaria case report form increased slightly in 2013 compared with 2012, but still remains unacceptably low. This incomplete reporting compromises efforts to examine trends in malaria cases and prevent infections. VFRs continue to be a difficult population to reach with effective malaria prevention strategies. Evidence-based prevention strategies that effectively target VFRs need to be developed and implemented to have a substantial impact on the numbers of imported malaria cases in the United States. Fewer patients reported taking chemoprophylaxis in 2013 (32%) compared with 2012 (34%), and adherence was poor among those who did take chemoprophylaxis. Proper use of malaria chemoprophylaxis will prevent the majority of malaria illness and reduce the risk for severe disease (http://www.cdc.gov/malaria/travelers/drugs.html). Malaria infections can be fatal if not diagnosed and treated promptly with antimalarial medications appropriate for the patient’s age and medical history, the likely country of malaria acquisition, and previous use of antimalarial chemoprophylaxis. Recent molecular laboratory advances have enabled CDC to identify and conduct molecular surveillance of antimalarial drug resistance markers (http://www.cdc.gov/malaria/features/ars.html). These advances will allow CDC to track, guide treatment, and manage drug resistance in malaria parasites both domestically and globally. For this to be successful, specimens should be submitted for all cases diagnosed in the United States. Clinicians should consult the CDC Guidelines for Treatment of Malaria and contact the CDC’s Malaria Hotline for case management advice, when needed. Malaria treatment recommendations can be obtained online (http://www.cdc.gov/malaria/diagnosis_treatment) or by calling the Malaria Hotline (770-488-7788 or toll-free at 855-856-4713).
Introduction
Malaria in humans is caused by infection with one or more of several species of Plasmodium (i.e., P. falciparum, P. vivax, P. ovale, P. malariae, and occasionally other Plasmodium species) parasites. The parasite is transmitted by the bite of an infective female Anopheles mosquito. P. falciparum and P. vivax species cause the most infections worldwide. P. falciparum is the agent that most commonly causes severe and potentially fatal malaria (see Definitions). An estimated 198 million clinical cases and 584,000 (0.3%) deaths were reported worldwide in 2013, mostly among children aged <5 years living in sub-Saharan Africa (1). P. vivax and P. ovale have dormant liver stages, which can reactivate and cause malaria several months or years after the initial infection. P. malariae can result in long-lasting infections and, if untreated or inadequately treated, can persist asymptomatically in the human host for years, even a lifetime (2). Approximately half of the world’s population live in regions where malaria is transmitted (i.e., approximately 100 countries in parts of Africa, Asia, the Middle East, Eastern Europe, Central and South America, the Caribbean, and Oceania) (2). Before the 1950s, malaria was endemic throughout the southeastern United States; an estimated 600,000 cases occurred in 1914 (3). During the late 1940s, a combination of improved housing and socioeconomic conditions, environmental management, vector-control efforts, and case management was successful at interrupting malaria transmission in the United States (4).* Since then, malaria case surveillance has been maintained to detect locally acquired cases that could indicate instances of local transmission, to monitor patterns of resistance to antimalarial drugs, and to guide malaria prevention recommendations for international travelers. Malaria vector mosquitoes are still present in the United States (5).
The majority of reported malaria cases diagnosed each year in the United States are imported from regions where mosquitoborne malaria transmission is known to occur, although congenital infections and infections resulting from exposure to blood or blood products also are reported in the United States (6). In addition, rare cases of local mosquitoborne transmission have been reported (7). State and local health departments and CDC investigate reported malaria cases in the United States, and CDC analyzes data from imported cases to detect trends in acquisition.
The signs and symptoms of malaria illness are varied, but the majority of patients have fever (8). Other common symptoms include headache, back pain, chills, increased sweating, myalgia, nausea, vomiting, diarrhea, and cough. A diagnosis of malaria should always be considered for persons with these symptoms who have traveled to an area with known malaria transmission. Malaria also should be considered in the differential diagnosis of persons who have fever of unknown origin, regardless of their travel history. Untreated infections can rapidly progress to coma, renal failure, respiratory distress, and death. This report summarizes malaria cases reported to CDC among persons with onset of symptoms in 2013.
Methods
Data Sources and Analysis
Malaria case data were reported to the National Malaria Surveillance System (NMSS) and the National Notifiable Diseases Surveillance System (NNDSS) (9). Although both systems rely on passive reporting, the numbers of reported cases might vary because of differences in collection and transmission of data. A substantial difference between the data collected in these two systems is that NMSS receives more detailed clinical and epidemiologic data regarding each case (e.g., information concerning the area to/from which the infected person has traveled). Malaria cases can be reported to CDC through either NMSS or NNDSS or through a direct consultation with CDC malaria staff; therefore, cases reported through these various paths are compared, unduplicated, compiled, and analyzed. The Armed Forces Health Surveillance Center (AFHSC) provided information about additional military cases that were not reported to state health departments, and those were added to the NMSS database. This report presents data on the aggregate of cases reported to CDC through all reporting systems.
Malaria cases are classified as confirmed or suspected using the 2009 Council of State and Territorial Epidemiologists (CSTE)/CDC case definition (10). Malaria cases are further categorized by infecting species: Plasmodium falciparum, P. vivax, P. malariae, and P. ovale. When more than a single species is detected, the case is categorized as a mixed infection. All categories are mutually exclusive. Diagnosis of malaria is made by blood film microscopy or polymerase chain reaction (PCR). A rapid diagnostic test (RDT) can be used to detect malaria antigens; however, the diagnosis must be confirmed by either microscopy or PCR to be counted as a case (i.e., only confirmed cases are included in this report). Each malaria case is reported by health care providers or laboratories to local or state health departments and to CDC. CDC staff review all reports, when received, and request additional information from the provider or the state, if necessary (e.g., when no recent travel is reported to or from a country where malaria is endemic). Reports of other cases are telephoned to CDC directly by health care providers, usually when they are seeking assistance with diagnosis or treatment. Information regarding cases reported directly to CDC is shared with the relevant state health department. All cases that have been reported as acquired in the United States are investigated further, including all induced, congenital, introduced, and cryptic cases (see Definitions). Information derived from uniform case report forms is entered into a database and analyzed annually (http://www.cdc.gov/malaria/resources/pdf/report/malaria_form.pdf).
The chi-square test was used to calculate p values and assess differences between variables reported in 2012 compared with previous years. A p value of <0.05 was considered statistically significant. Linear regression using least-squares methods was used to calculate the average increase in the number of cases since the early 1970s.
Definitions
The following definitions are used in malaria surveillance for the United States:
-
U.S. residents — Persons residing in the United States, including both civilian and U.S. military personnel, regardless of legal citizenship.
-
U.S. civilians — Any U.S. residents, excluding U.S. military personnel.
-
Foreign residents — Persons who hold resident status in a country other than the United States.
-
Travelers visiting friends or relatives — Immigrants, ethnically and racially distinct from the major population of the country of residence (a country where malaria is not endemic), who return to their homeland (a country where malaria is endemic) to visit friends or relatives. Included in the visiting friends and relatives (VFR) category are family members (e.g., spouse or children) who were born in the country of residence.
-
Laboratory criteria for diagnosis: Demonstration of malaria parasites on blood film, PCR, or by RDT (followed by blood film confirmation).
-
Confirmed case: Symptomatic or asymptomatic infection that occurs in a person in the United States or one of its territories who has laboratory-confirmed (by microscopy or PCR) malaria parasitemia, regardless of whether the person had previous episodes of malaria while in other countries. A subsequent episode of malaria is counted as an additional case, regardless of indicated Plasmodium species, unless the case is indicated as a treatment failure resulting from drug resistance.
-
Suspect case: Symptomatic or asymptomatic infection that occurs in a person in the United States or one of its territories who has Plasmodium species detected by rapid diagnostic antigen testing without confirmation by microscopy or PCR, regardless of whether the person experienced previous episodes of malaria while in other countries.
-
Partial immunity: Immunity in persons born in malaria endemic areas who have survived multiple infections with malaria. Although these persons remain susceptible to malaria, their subsequent infections, however, are less likely to be severe. This protection from severe malaria wanes if the person is no longer exposed to repeated malaria infections. Several antibodies have been identified that are a part of the immune response to malaria, but no test can classify persons as immune or not.
This report also uses terminology derived from the recommendations of the World Health Organization (11). Definitions of the following terms are included for reference:
-
Autochthonous malaria:
-
Indigenous. Mosquitoborne transmission of malaria in a geographic area where malaria occurs regularly.
-
Introduced. Mosquitoborne transmission of malaria from a person with an imported case in an area where malaria does not occur regularly.
-
-
Imported malaria: Malaria acquired outside a specific area. In this report, imported cases are those acquired outside the United States and its territories.
-
Induced malaria: Malaria acquired through artificial means (e.g., blood transfusion, organ transplantation, or by using shared syringes).
-
Relapsing malaria: Recurrence of disease after it has been apparently cured. In malaria, true relapses are caused by reactivation of dormant liver-stage parasites (hypnozoites) of P. vivax and P. ovale.
-
Severe malaria: A case of malaria with one or more of the following manifestations: neurologic symptoms, renal failure, severe anemia (defined by hemoglobin [Hb] <7g/dL), acute respiratory distress syndrome (ARDS), jaundice, or ≥5% parasitemia (12). To attempt to include severe cases in which clinical criteria were not reported, persons who were treated for severe malaria (i.e., artesunate, quinidine, and/or an exchange blood transfusion) despite having no specific severe manifestations reported also are counted as a severe case in this analysis.
-
Cryptic malaria: A case of malaria for which epidemiologic investigations fail to identify a plausible mode of acquisition (this term applies primarily to cases found in countries where malaria is not endemic).
Laboratory Diagnosis of Malaria
To diagnose malaria promptly, physicians must obtain a travel history from every febrile patient. Malaria should be included in the differential diagnosis of every febrile patient who has traveled to a malarious area. If malaria is suspected, a Giemsa-stained film of the patient’s peripheral blood should be examined for parasites as soon as possible. Thick and thin blood films must be prepared correctly because diagnostic accuracy depends on blood film quality and examination by experienced laboratory personnel (13). This simple test can quickly detect the presence of malaria parasites and can also be used to determine the species and percentage of red blood cells that are infected, which are all essential to guiding appropriate treatment of persons infected with malaria. During the Ebola outbreak in West Africa that began in 2014, laboratories expressed concern that the Ebola virus might not be inactivated by the smear preparation process. As a result, CDC developed additional steps to inactivate viruses including Ebola during the slide preparation process (14). Some reference laboratories and health departments can diagnose malaria using PCR, although this is generally reserved for cases for which blood film diagnosis of malaria is inadequate and for confirmation of species. PCR results are also often not available quickly enough to be of use in the initial diagnosis and treatment of a patient with malaria.
In addition, BinaxNOW Malaria, an RDT that detects circulating malaria-specific antigens, is approved for use by hospital and commercial laboratories. Therefore, the test should be used in a clinical laboratory by trained staff; it should not be used by clinicians or the general public (15,16). In the United States, use of RDTs can decrease the amount of time required to determine whether a patient is infected with malaria but does not eliminate the need for standard tests (16). RDTs are not able to speciate or quantify malaria parasites. Positive and negative RDTs must be confirmed by microscopy (6), which is necessary to provide the additional information about species and density of infection. If microscopy was not performed, a PCR result can also be used to confirm RDT result and determine the species.
Drug Resistance Marker Surveillance
In 2012, CDC’s Malaria Branch began molecular surveillance for malaria drug resistance markers. The goal is to evaluate cases of malaria diagnosed and treated in the United States as a means of detecting and characterizing malaria parasites that carry genetic markers (typically single nucleotide polymorphisms in one or more loci) associated with drug resistance. These data will help to understand where foci of resistance to different drugs might be present or emerging in specific parts of the world where malaria is endemic. For each sample submitted, species confirmation testing is conducted using a duplex real-time PCR capable of detecting the four human infecting Plasmodium species. For mixed infections, samples are also processed by nested-PCR using species-specific primers that accurately detect the minority population of the co-infecting malaria species. Molecular fingerprinting methods based on microsatellite markers and single nucleotide polymorphisms are used to identify antimalarial drug resistance markers for P. falciparum samples only at this time. Additional species will be similarly evaluated as new laboratory methods are developed. Each sample submitted is tested for molecular markers associated with resistance to chloroquine, sulfadoxine-pyrimethamine, mefloquine, atovaquone, and artemisinins.
The parasite DNA is subjected to PCR amplification using appropriate primers and sequenced using the Sanger method using the ABI 3130 capillary sequencer according to described methods (17). Fragments of genes encoding molecular targets of chloroquine (chloroquine resistance transporter gene, pfcrt), pyrimethamine (dihydrofolate reductase gene, dhfr), sulfadoxine (dihydropteroate synthase gene, dhps), atovaquone (cytochrome b gene, cytb), mefloquine (multidrug resistance 1 protein gene, pfmdr-1 and pfmdr-1 copy number), and artemisinin (Mal13–1718319) were analyzed for polymorphisms by comparing each sequence to the reference genome. All reactions were conducted in triplicate on a Stratagene MX3005P (Agilent Technologies) real-time PCR machine. Resistance genes were assessed for the following drugs: chloroquine, pyrimethamine, sulfadoxine, atovaquone, mefloquine, and artemisinins.
-
Chloroquine resistance markers. The pfcrt gene sequence was analyzed to identify polymorphism at codons C72S, M74I, N75E, and K76T.
-
Pyrimethamine resistance markers. The dhfr gene sequence was analyzed to identify polymorphism at codons A16V, C50R, N51I, C59R, S108T/N, and I164L.
-
Sulfadoxine resistance markers. The dhps gene sequence was analyzed to identify polymorphism at codons S436A, A437G, and K540E.
-
Atovaquone resistance markers. The cyto b gene sequence was analyzed to identify polymorphism at codons I258M and Y268S (18).
-
Mefloquine resistance markers. The pfmdr-1 gene sequence was analyzed to identify polymorphism at codons N86Y, Y184F, S1034C, N1042D, and D1246Y.
-
pfmdr-1 copy number. A real-time PCR assay was used to determine the copy number of pfmdr-1 relative to that of a single copy gene, seryl-T synthetase, using the comparative cycle threshold (ΔΔCT) method (19). The measured copy number of the pfmdr-1 gene relative to that of a standard calibrator parasite, 3D7, which has a single copy of pfmdr-1. In addition, DNA from Indochina W2 and Dd2 was used as multiple copy number controls.
-
Artemisinin resistance markers: Pyrosequencing was used to test for artesmisinin resistance at previously reported (20) polymorphisms located on chromosome 10 (MAL10–688956) and chromosome 12 (MAL13–1718319) that are associated with artemisinin resistance in P. falciparum parasites. Recently, another artemisinin resistance gene called kelch k13-propeller domain containing gene was reported (21). The k13-propeller domain was amplified using a nested PCR method previously described (21,22). The sequence data was analyzed using Geneious Pro R8 to identify polymorphisms associated with artemisinin resistance.
Resistance was then classified into levels on the basis of the number of accumulated mutations detected. Samples classified as sensitive demonstrated no mutations. For chloroquine, mefloquine, atovaquone, and artemisinin, resistance was defined as having detected any mutations. For pyrimethamine and sulfadoxine, resistance was defined as low if one mutation was detected, moderate if two mutations were detected, and high if three or more mutations were detected.
Results
General Surveillance
In 2013, CDC received 1,727 reports concerning cases of malaria among persons in the United States and its territories, representing a 2% increase from the 1,687 cases reported with onset of symptoms in 2012. Since 1973, the trend has been increasing in the total number of cases of malaria reported in the United States. On average, 28.8 additional cases are reported in the United States each year since 1973 (Figure 1). In 2013, a total of 1,137 cases occurred among U.S. residents, 348 cases among foreign residents, and 242 cases among patients with unknown or unreported resident status (Table 1).
Plasmodium Species
Among the 1,727 cases reported in 2013, the infecting species of Plasmodium was identified and reported in 1,441 (83%) cases. Of the 1,441 cases, 104 had specimens submitted to CDC for species confirmatory testing. Overall, the proportion of cases with complete reporting of species was equal to that for 2012 (83%) (Table 2) (6). P. falciparum and P. vivax comprised the majority of infections and were identified in 73% and 17% of 1,441 infected persons with species reported, respectively. The percentage of identified cases that were P. vivax decreased approximately three percentage points from 2012. Among 1,349 cases for whom both the region of acquisition and the infecting species were known, P. falciparum accounted for 85% of infections acquired in Africa, 73% in Central America and the Caribbean, 24% in South America, 14% in Oceania, and 9% in Asia (Table 3). In Central America and the Caribbean, the proportion of infections that were caused by P. falciparum decreased by 7 percentage points compared with 2012. This was likely a result of a decrease in the total number of cases reported both across the region and from Haiti. Cases reported from Haiti decreased from a high of 171 in 2010 (23) to 22 in 2013. Infections attributed to P. vivax accounted for 86% acquired in Oceania, 80% in Asia, 67% in South America, 22% in Central America and the Caribbean, and 5% in Africa.
Region of Acquisition and Diagnosis
Among the 1,727 reported cases, two congenital cases were reported and five cases did not have information reported to allow for their importation status to be determined. A total of 1,720 reported cases were classified as imported. Information on region of acquisition was missing for 203 (12%) imported cases. Of 1,517 imported cases for which the region of acquisition was known, 1,250 (82%) were acquired in Africa, 164 (11%) in Asia, 41 (3%) in Central America and the Caribbean, 53 (3%) in South America, eight (1%) in Oceania, and one (<1%) in Europe (Table 3). Countries in West Africa† accounted for 832 (67%) cases acquired in Africa. Although the overall percent of cases acquired in West Africa remained unchanged from 2012, the distribution of cases within Africa changed; decreases in the number of cases acquired in Sudan (62 in 2012 and 41 in 2013) and Ethiopia (41 in 2012 and 29 in 2013) were balanced out by increases in the number of cases acquired in Cameroon (31 in 2012 and 63 in 2013), Liberia (100 in 2012 and 130 in 2013), and Nigeria (244 in 2012 and 265 in 2013). In Asia, the number of cases that were acquired in South Asia§ decreased significantly from 183 in 2012 to 147 in 2013; however, India was in the top five countries of acquisition. A decrease was observed in Afghanistan (79% reduction from 28 in 2012 to six in 2013) as a result of decreased cases among U.S. military personnel serving in Afghanistan. Four of the six cases acquired in Afghanistan were among U.S. military personnel. A 40% decrease occurred in cases acquired from Central America and the Caribbean in 2013 compared with 2012, attributed entirely to the 37% decrease in cases from Haiti (35 in 2012 and 22 in 2013). The number of cases acquired in South America was not notably different from 2011 (35 in 2011, 41 in 2012, and 53 in 2013), with the majority of cases acquired in Guyana and Peru (Table 3).
In the United States, eight reporting areas accounted for 53% of the 1,727 reported malaria cases: New York City (n = 223), Maryland (n = 158), California (n = 115), New Jersey (n = 103), Texas (n = 91), Pennsylvania (n = 78), Virginia (n = 76), and Massachusetts (n = 75) (Figure 2). The states with the largest increase in reported malaria cases in 2013 were New Jersey, which increased by 61% to return to 2011 levels (104 in 2011, 64 in 2012, and 103 in 2013), and Maryland, which increased by 28% from 2012 (123 in 2012 versus 158 in 2013). The states with the largest decrease in reported malaria cases in 2013 were Missouri with a 76% decrease (25 in 2012 versus six in 2013) and Texas with a 17% decrease (110 in 2012 versus 91 in 2013).
Imported Malaria by Resident Status
Among the 1,484 imported malaria cases of known resident status, 1,136 (77%) occurred among U.S. residents and 348 (23%) among residents of other countries. Among the 1,136 imported malaria cases among U.S. residents, 941 (83%) were acquired in Africa, 89 (8%) in Asia, 38 (3%) in South America, 32 in Central America and the Caribbean (3%), and eight in Oceania (1%) (Table 4). This represents a significant 3% increase in cases among U.S. residents who acquired malaria in Africa compared with 2012 (921 in 2012 versus 941 in 2013) and a trend observed since 2008 (6,13,23–25). The number of cases acquired in Asia decreased from 2012 (111 in 2012 versus 89 in 2013), but this change was not statistically significant. Likewise, cases acquired in the Americas among U.S. residents decreased but not significantly compared with 2012 (82 in 2012 versus 70 in 2013). No significant change was noted in the cases acquired in Oceania between 2012 and 2013. Of the 348 imported cases among foreign residents, 247 (71%) were acquired in Africa, 67 (19%) in Asia, 12 (3.4%) in South America, six (2%) in Central America and the Caribbean, and one (<1%) in Europe. The countries of acquisition with the most significant reduction in the reported number of cases of malaria in foreign residents were India and Ghana, whereas cases acquired in Nigeria, Guyana, Liberia, and Cameroon increased among foreign residents. Among 239 foreign residents for whom purpose of visit to the United States was known, 68 (28%) were among VFRs and 126 (53%) occurred in recent immigrants or refugees, among whom 102 (81%) were from Africa.
Seasonality of Malaria Diagnosed in the United States
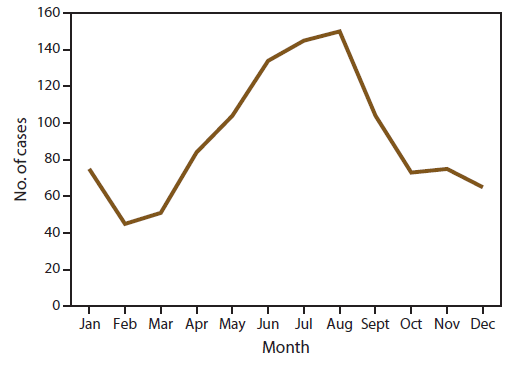
The total number of cases reported in the United States peaked in August and occurred primarily among persons who indicated travel to Africa (Figure 3). This likely correlated with peak travel times to African destinations related to summer holidays (26). The seasonality observed was attributed to the pattern of occurrence among P. falciparum cases; the number of P. vivax cases reported in the United States also peaked in August, but that peak was much smaller than for P. falciparum. Most of those cases were among persons who indicated travel to Asia (most of whom had traveled to India).
Interval Between Arrival in the United States and Illness Onset
Among the 1,434 imported malaria cases with an identified Plasmodium species, the interval between the date of arrival in the United States and onset of illness was known for 980 (68%) cases (Table 5). Onset of symptoms began before arrival in the United States in 120 (12%) cases; the remaining 860 (88%) patients experienced malaria symptoms on or after arrival to the United States. Onset of malaria symptoms occurred 0–29 days after arrival in 620 (81%) of the 766 P. falciparum cases and in 60 (45%) of the 133 P. vivax cases.
Imported Malaria Among U.S. Military Personnel
In 2013, a total of 14 cases of imported malaria were reported among U.S. military personnel, a significant decrease from the 92 reported in 2011 and the 43 reported in 2012. Region of travel was known for 11 cases and unspecified for three cases. Four military personnel reported travel to Afghanistan and four to various regions in Africa. One each reported travel to multiple countries in Central America and two reported travel to South Korea. Compared with 2012, fewer military personnel reported travel to Afghanistan and none reported travel to Haiti, likely as a result of changes in U.S. military presence in those regions (27). Information on infecting species was known for 13 cases; nine cases were identified as P. vivax, two as P. falciparum, and one each of P. malariae and P. ovale. Among those 13 cases, only six occurred in persons who reported having taken at least 1 dose of an appropriate drug for primary chemoprophylaxis; only one reported adhering to the regimen. Among the nine military personnel infected with P. vivax, only two reported treatment with primaquine to avoid future relapses. Only one of the 14 cases was classified as severe; it was caused by P. vivax infection.
Chemoprophylaxis Use Among U.S. Civilians
Information about chemoprophylaxis use and travel area was known for 956 (85%) of the 1,122 U.S. civilians who had imported malaria. Of these 956 persons, 308 (32%) took chemoprophylaxis. Among the 308 persons who reported taking malaria chemoprophylaxis, 90 (29%) did not report a specific drug type taken. Of the remaining 218 persons, 184 (84%) took a CDC-recommended medication and 22 (10%) took a medication that is not recommended by CDC for the area visited. Of the 184 who reported taking CDC-recommended chemoprophylaxis, 52 (28%) took mefloquine, 80 (43%) took doxycycline, 38 (21%) took atovaquone/proguanil, one (<1%) took chloroquine, none took primaquine, and 13 (7%) took more than one CDC-recommended malaria chemoprophylaxis medication for the specific travel region. Information about infecting species was available for 162 (88%) patients who took a recommended antimalarial drug and was undetermined for the remaining 22 patients. Moreover, among the 152 who reported taking CDC-recommended chemoprophylaxis and for whom adherence was known, 110 (72%) reported nonadherence (i.e., missed doses).
Cases of P. vivax or P. ovale After Recommended Prophylaxis Use. Among the 162 patients who took a recommended medication for chemoprophylaxis and had information on infecting species, 21 (13%) cases were caused by P. vivax, and 15 (9%) cases were caused by P. ovale. Of the 36 cases of P. vivax or P. ovale, information on 16 cases was insufficient (i.e., missing data regarding symptom onset or return date from travel) to assess if this was an acute infection or a relapse infection. Onset of symptoms for nine reported cases occurred >45 days after the patient arrived in the United States. The clinical features of these cases are consistent with relapsing infections and do not indicate primary prophylaxis failures. Eleven cases occurred ≤45 days after the patient returned to the United States; these cases are consistent with acute infection and could indicate primary prophylaxis failures. Among the 11 cases, six patients were nonadherent with their malaria chemoprophylaxis regimen, and two patients did not provide adherence information. The remaining three patients reported adherence with an antimalarial chemoprophylaxis regimen. Two patients who reported adherence to the chemoprophylaxis regimen had traveled to Africa; one took only atovaquone/proguanil for malaria chemoprophylaxis and the other reported taking mefloquine and doxycycline. The other patient traveled to Papua New Guinea for tourism and reported taking doxycycline. Possible explanations for infection in these patients include inappropriate dosing, unreported nonadherence, malabsorption of the drug, an early relapse from hypnozoites established at the start of the trip, or possibly emerging parasite resistance.
Cases of P. falciparum or P. malariae After Recommended Prophylaxis Use. The 162 cases of malaria reported among persons who took a recommended antimalarial drug for chemoprophylaxis included 116 cases of P. falciparum, five cases of P. malariae, and five cases with mixed infection. Of the 116 P. falciparum cases, 113 (97%) were acquired in Africa, one (1%) in Haiti, and two (2%) in Guyana. Seventy-eight (67%) of the 116 P. falciparum patients reported nonadherence to the antimalarial drug regimen, 23 (20%) patients reported adherence, and 15 patients had no adherence information available. Of the 23 cases in which patients reported adherence with antimalarial chemoprophylaxis, one traveled to Guyana and took mefloquine for malaria chemoprophylaxis and 22 had traveled to Africa. Of those who traveled to Africa, eight patients took atovaquone/proguanil, eight took mefloquine, five took doxycycline, and one reported taking both atovaquone/proguanil and artemether/lumefantrine. Of the five P. malariae cases, only one reported adherence to the antimalarial drug regimen; the patient traveled to Guinea and reported taking mefloquine.
Patients with a Recent History of Malaria
Of the 1,720 imported cases, data on history of malaria was known for 1,083 (63%) cases; 225 (21%) patients reported a history of malaria infection during the preceding 12 months. Among the 225 cases, 107 were P. falciparum (48%), 51 (23%) were P. vivax, 20 (9%) were P. ovale, six (3%) were P. malariae, six (3%) were mixed infections, and 35 (16%) reported no species. A total of eight probable relapses were identified on the basis of onset date, date of previous infection, and previous infection species: six P. vivax cases and two P. ovale cases. Among the eight relapses, only three patients (two were P. vivax infections and one was a P. ovale infection) subsequently received primaquine as part of treatment to avoid future relapses.
Purpose of Travel
Purpose of travel to regions in which malaria is endemic was reported for 904 (81%) of the 1,122 U.S. civilians with imported malaria (Table 6). Of the 904 who reported purpose of travel, 635 (70%) were VFRs, 69 (8%) were missionaries, and 92 (10%) were traveling for business. The proportion of VFRs among 1,122 U.S. civilians with imported malaria increased (66% in 2012 versus 70% in 2013), and the proportion of military travelers decreased (4% in 2012 versus 1% in 2013).
Malaria by Age
Among the 1,720 imported malaria cases among patients for whom age was known, 291 (17%) occurred in persons aged <18 years, 1,325 (77%) in persons aged 18–64 years, and 104 (6%) in persons aged ≥65 years. Although the majority of cases occurred in persons aged 18–64 years, pediatric cases are of particular interest because the preventive care of most children is determined by parents or guardians. Among the 291 cases among persons aged <18 years, 166 (57%) occurred among U.S. civilian children, 101 (35%) occurred among children of persons categorized as having a foreign resident status at the time their malaria infection was acquired, and 24 (8%) occurred among children of unknown resident status. Of the 166 cases among U.S. civilian children, nine (5%) were aged <2 years, 30 (18%) were aged 2–4 years, 68 (41%) were aged 5–12 years, and 59 (36%) were aged 13–17 years. A total of 140 (88%) of the cases among 159 U.S. civilian children for whom country of exposure was known were attributable to travel to Africa. Among the 137 U.S. civilian children for whom reason for travel was known, 113 (82%) were VFRs, 11 (8%) were traveling for educational purposes, eight (6%) were traveling for missionary work, four (3%) were traveling for tourism, and one (1%) accompanied their parent/guardian on a business trip. Of the 151 children for whom chemoprophylaxis information was known, 64 (42%) were reported as having taken chemoprophylaxis, of whom 27 (42%) had taken an appropriate regimen; however, only 11 (41%) of these 27 patients reported adherence.
Hospitalization
Information on hospitalization was reported for 1,450 (84%) cases. Among those persons, 1,003 (69%) were hospitalized. The majority of those cases were P. falciparum (n = 710 [71%]), of which 213 (30%) were considered severe. This represents a significant increase in the number of severe cases of P. falciparum who were hospitalized (160 in 2012 versus 213 in 2013). The second largest proportion of hospitalized cases were P. vivax (n = 124 [12%]). The majority of hospitalized P. vivax patients had uncomplicated malaria infections; however, seven (6%) were severe.
Treatment in Uncomplicated Imported Malaria Cases
Of the 1,450 imported cases of uncomplicated malaria in 2013, information on treatment medicines was available for 1,031 (71%) persons. This represents a significant decrease in the number of cases with available treatment information (77% in 2012 versus 71% in 2013). Of these, 643 (62%) were P. falciparum, 172 (17%) P. vivax, 48 (5%) P. ovale, 25 (2%) P. malariae, 27 (3%) mixed cases, and 116 (11%) were a species type that was unknown or not reported. The CDC Guidelines for Treatment of Malaria in the United States, herein referred to as the CDC Guidelines for Treatment, was used to determine whether the medicines listed for treatment were appropriate (28).
Of the 1,031 patients with uncomplicated malaria with available information on treatment, 868 (84%) were treated appropriately according to the CDC Guidelines for Treatment, and 163 (16%) patients received inappropriate treatment. The percent of patients with uncomplicated disease who were treated appropriately was unchanged from 2012. Among the patients who were treated appropriately, 114 (13%) indicated taking other antimalarial drugs in addition to those recommended by CDC guidelines. Because the CDC surveillance report form does not record the sequence of treatment events, it is difficult to understand and characterize the intended purpose of additional antimalarial treatment drugs. Therefore, for the purpose of this report, these 114 patients were considered to be treated appropriately. Among the 163 inappropriately treated patients, 13 (8%) had received a recommended antimalarial for chemoprophylaxis but subsequently had inappropriately received the same drug for treatment. Antimalarial drugs used for treatment should differ from the drugs received for chemoprophylaxis because of the potential for toxicity and reduced efficacy.
Adequacy of treatment varied by species. For the 643 P. falciparum cases, 551 (86%) patients were treated appropriately, according to the CDC Guidelines for Treatment, of which 85 (15%) received additional antimalarial drugs. The 92 P. falciparum cases that were treated with an inappropriate treatment regimen included four pregnant patients. Among the 25 P. malariae cases, 22 (88%) patients were treated appropriately according to the CDC Guidelines for Treatment, of whom three (14%) received other antimalarial drugs in addition to those recommended by CDC. Three (12%) patients infected with P. malariae were treated with an inappropriate treatment regimen.
Among the 172 patients with P. vivax for whom treatment information was reported, 145 (84%) were treated with an appropriate antimalarial drug to address their acute infection, of which six (4%) received other antimalarial drugs in addition to those recommended by CDC. Of the 145 P. vivax cases who received an appropriate treatment for their acute infection, less than half (n = 63 [43%]) were also treated with primaquine for relapse prevention, which CDC recommends for all cases of mosquito-acquired P. vivax infections. Among the 48 patients with P. ovale for whom treatment information was reported, 44 (92%) were treated with an appropriate antimalarial drug to address their acute infection, of whom three (7%) received other antimalarial drugs in addition to those recommended by CDC. Of the 44 P. ovale patients who received an appropriate treatment for their acute infection, 13 (30%) also were treated with primaquine for relapse prevention. Among the 27 mixed cases for whom treatment information was reported, 19 (70%) patients were treated appropriately, according to the CDC Guidelines for Treatment. Six of those received other antimalarial drugs in addition to the CDC-recommended regimens. Of the eight mixed cases that were not treated appropriately, six included at least one relapsing species and only two received primaquine.
According to the CDC Guidelines for Treatment, when species is unknown, a treatment regimen for a P. falciparum infection should be used to treat infection. Among the 116 cases where species was unknown, 87 (75%) patients were treated appropriately according to the CDC Guidelines for Treatment, of whom 11 (13%) received other antimalarial drugs, in addition to those recommended by CDC. Twenty-nine (25%) patients received an inappropriate treatment regimen. Incomplete reporting of species and treatment medications might affect whether the case is classified as having been treated appropriately or not.
Severe Malaria
Among the 1,727 reported cases, 270 (16%) were classified as severe malaria, including 10 cases in which patients died. Most (199 [74%]) severe cases occurred in persons aged ≥18 years, and 70 (26%) occurred in children aged <18 years, 13 (19%) of whom were aged <3 years. Age was not reported for one (<1%) patient. Persons aged <5 years were significantly more likely to have severe disease when compared with those ≥5 years (37% of those <5 years versus 15% of those ≥5 years). No association was found between severe disease and resident status. Among the 260 cases in patients with known resident status, 201 (77%) were U.S. residents. The predominant species among the severe cases was P. falciparum (n = 233 [86%]), which was significantly higher than 2012 (n = 174 [75%]).
Where information on prophylaxis was known (n = 230), 47 (20%) persons reported taking a recommended chemoprophylaxis; however, only 11 reported adherence to the drug regimen, including five who took doxycycline, three who used mefloquine, and three who took atovaquone/proguanil. One of the three persons who reported taking atovaquone/proguanil also reported taking artemether/lumefantrine. Patients with severe cases were less likely to have taken prophylaxis than those with uncomplicated cases (20% versus 30%). Although some patients had multiple clinical complications associated with their infection, the largest proportion of patients experienced renal failure (18%), followed by severe anemia (Hb <7g/dL) (16%), cerebral malaria (11%), ARDS (9%), and jaundice (5%). Patients with severe disease were more likely to receive inappropriate treatment than those with uncomplicated disease. Among the 270 severe cases, 149 (55%) patients were treated with quinidine and 81 (30%) were treated with an oral antimalarial drug. Forty-three (16%) patients were treated with IV artesunate provided by CDC through an investigational new drug (IND) protocol. Patients diagnosed with uncomplicated malaria can be effectively treated with oral antimalarial drugs. However, patients who are considered to have severe disease should be treated aggressively with parenteral antimalarial therapy (28).
No significant difference was observed in the number of days from date of arrival in the United States to the date of hospitalization between severe and uncomplicated P. falciparum cases (17.0 days for severe and 12.7 days for uncomplicated). In addition, no significant difference was observed in the length of time between date of onset of illness and date of hospitalization between severe and uncomplicated P. falciparum cases (6.5 days for severe and 5.8 days for uncomplicated). The dates on which patients first saw a medical provider were not collected, so time from first provider encounter to hospitalization could not be examined.
No association existed between the reason for travel and severe malaria. Among patients for whom reason for travel was known, most (63%) of the severe cases were in VFRs (comparable with 2011), of whom 74% specified acquisition from West Africa; 89% of severe cases were identified as P. falciparum infections. Three (1%) of the severe cases were acquired in Haiti, significantly below 2011 levels when 5% of the severe cases were acquired in Haiti. In Haiti, virtually all malaria is caused by P. falciparum.
Malaria During Pregnancy
A total of 36 cases of malaria were reported among pregnant women in 2013, representing 6% of cases among all women (n = 639). The number of pregnant women with malaria did not change significantly from the 34 cases reported in 2012. In addition, no significant differences were noted among pregnant women with malaria compared with nonpregnant women, in terms of species type, reason for travel, or region of infection acquisition. Of the 36 cases among pregnant women, eight (22%) cases were severe, all of whom were hospitalized and survived. Among the 29 cases for whom Plasmodium species type was known, 22 (76%) were diagnosed with P. falciparum infection, including the eight patients who presented with severe malaria. Two (7%) were diagnosed with P. vivax, two with P. malariae, one with P. ovale, two with mixed infections, and seven (19%) with no species reported. Twenty-two of the 36 (61%) cases occurred among pregnant women who were U.S. civilians; most of whom (91%) reported travel to Africa. Among the 15 U.S. civilian pregnant women with known reason for travel, 93% were VFRs. Of the 22 cases of malaria reported among U.S. civilian pregnant women, seven (32%) reported taking malaria chemoprophylaxis. None of the seven women reported adhering to their chemoprophylaxis. No information was available on birth outcomes.
Drug Resistance Markers
In 2013, a total of 137 blood samples were sent to CDC and tested for genetic markers associated with resistance to antimalarial drugs. Because the current focus for drug resistance is for P. falciparum, CDC first determines the species by PCR. All other species are archived for future testing. Twenty-nine state and local health departments submitted samples for testing. Species of malaria parasite was confirmed by PCR to be P. falciparum in 100 (73%) samples, followed by P. vivax (15 [11%]), P. ovale (14 [10%]), P. malariae (six [4%]), and mixed infections (two [1%]). PCR determined or corrected the species for 85 samples (62%) submitted (Table 7). Of the P. falciparum-positive samples with results, 88 (93%) had genetic polymorphisms associated with pyrimethamine drug resistance, 74 (76%) with sulfadoxine resistance (37 with one, 22 with two, and 15 with three or more resistance markers), 53 (53%) with chloroquine resistance, none with mefloquine resistance, one (1%) with atovaquone resistance, and none with artemisinin resistance (Table 8). Of the P. falciparum-positive patients that reported location of recent travel, most reported recent travel to Africa. In addition, one case of atovaquone/proguanil resistance was detected in a U.S. worker returning from Nigeria in whom resistance developed during treatment and genetic testing confirmed the presence of resistance markers (29). Another case was described in 2012 (24).
Selected Malaria Case Reports
Congenital Cases
Two congenital cases caused by transmission of parasites from mother to child during pregnancy or during labor and delivery were reported in 2013. Clinical and laboratory data are limited.
Case 1. In June, a male term infant aged 3 weeks developed a fever of 102°F (38°C), rigors, and overall fussiness. He was seen in the hospital where P. vivax was diagnosed by smear microscopy and RDT. His mother had immigrated to the United States from Ethiopia 6 months earlier and reported having been diagnosed with and treated for malaria with unspecified medication when she was 2 months pregnant. She also reported having a fever 1 week before delivery that resolved without treatment. The baby was treated with chloroquine. The hospital staff indicated their intention to test the mother for G6PD deficiency and, if negative, treat her with primaquine. The baby had no clinical complications and recovered fully.
Case 2. In July, a male infant of unknown gestation, aged 1 day, had P. falciparum diagnosed with a parasitemia of 0.1%. The same day, his mother also had P. falciparum diagnosed with a 2% parasitemia. She had come to the United States from Nigeria 1 week before delivery. The mother was initially treated with atovaquone/proguanil, but her doctor switched her to quinine and clindamycin to complete treatment. The infant was treated with atovaquone/proguanil. Both mother and infant had no clinical complications and recovered fully.
Fatal Cases
Case 1. In June, a man aged 64 years returned to the United States after a 10-day trip to Ghana. He had not used chemoprophylaxis while in Ghana. He developed a fever 1 week after returning to the United States but did not seek care for 3 more days. On July 5, when he sought medical care, he was empirically treated for fever with doxycycline and azithromycin. The following day he developed confusion and focal neurologic deficits. Soon after arrival at the hospital on July 6 (4 days after symptom onset), he became comatose and was intubated and placed on mechanical ventilation in the intensive care unit (ICU). P. falciparum malaria was diagnosed by microscopy and RDT on the evening of July 6, but parasitemia was not initially calculated; he was prescribed oral quinine and intravenous (IV) doxycycline. A CT showed evidence of severe cerebral edema and impending brainstem herniation. The following morning, his thin blood film revealed a 50% parasitemia. CDC was contacted and artesunate was provided under the IND protocol. The patient received three IV doses of artesunate starting on July 7. A subsequent malaria blood film was obtained; which noted that malarial parasites were present, but the parasitemia was not quantified. On the second day of his hospitalization, a nuclear medicine scan revealed no cerebral circulation and an EEG showed no cortical activity. The decision was made to withdraw support, and he died on July 8.
Case 2. In late April, a man aged 75 years returned from a 3-month visit to Ghana to see family and friends. He was originally from Ghana but had been a resident of the United States for many years. He had a medical history of atrial fibrillation, hypertensive heart disease, and hypercholesterolemia. He reported taking mefloquine for prophylaxis, but it was not known whether he was adherent. Approximately 2 months after returning to the United States, he reported onset of fatigue. Two weeks later he developed a subjective fever. Three days later, he went to the emergency department, where he was found to have a fever of 102°F (38°C), mild anemia, and thrombocytopenia. The following day, P. falciparum was diagnosed by microscopy with 4.7% parasitemia. He was immediately started on IV quinidine and doxycycline in the ICU. His parasitemia dropped to 1.8% by the second day of his hospitalization and steadily declined until no further parasites were visualized by microscopy on day 8. On hospital day 6, he developed right upper lobe pneumonia and respiratory distress, necessitating intubation with mechanical ventilation. His respiratory status never improved and was complicated by worsening heart and then renal failure. He died of these nosocomial complications (pneumonia, renal failure, and heart failure) 6 weeks after admission to the hospital.
Case 3. In January, a man aged 51 years returned to the United States after a 7-week business trip to Liberia, where he did not take malaria chemoprophylaxis. He developed a fever 4 days after return to the United States but did not seek medical attention. Six days after his onset of fever, he was found to have an altered mental status and was taken to an emergency department, where he was diagnosed with cerebral malaria by microscopy (P. falciparum 15% parasitemia), along with jaundice and severe acidosis. He was started on IV quinidine gluconate and IV doxycycline. In ICU, he developed quinidine-induced QTc interval prolongation and was switched to artesunate under the CDC IND protocol. His parasitemia decreased to 3% on day 2 of hospitalization. However, he remained severely acidotic with significant electrolyte abnormalities refractory to dialysis and IV bicarbonate. On February 6, he developed cardiac arrest, and resuscitation attempts were unsuccessful.
Case 4. In April, a man aged 37 years returned to the United States following a 3-week trip to Sierra Leone to visit friends and relatives. He had not taken malaria chemoprophylaxis. Five days after returning, he developed fatigue, malaise, and vomiting. He presented to an urgent care center, where he was found to have thrombocytopenia and leucopenia. He was referred to a hematologist but no appointment was made. His symptoms worsened over the next few days and he experienced changes in his mental status. On April 14, he was transported to the hospital by ambulance. He was unresponsive in the emergency department. After arrival, he had a seizure and was admitted to the ICU and intubated. A blood film was positive for infection with P. falciparum. His peak parasitemia was 31%. He was started on IV doxycycline and IV quinidine. He developed refractory hypotension and was switched to IV artesunate. He developed renal failure with lactic acidosis, severe cerebral edema, and a subarachnoid hemorrhage. A head CT 6 days after admission demonstrated cerebral herniation. Additional studies demonstrated no cerebral blood flow and no brain activity. He was declared brain dead, support was withdrawn, and he died on April 22.
Case 5. In late November, a man aged 65 years returned to the United States following a 3-week trip to Liberia, where he had visited friends and relatives. He had a history of hypertension, diabetes mellitus, and chronic renal insufficiency. He had not taken any malaria chemoprophylaxis while on his trip. He began experiencing chills when his return flight was landing and sought medical attention the following day. A diagnosis of malaria was not considered, and he was sent home with a prescription for doxycycline, which he did not take. His symptoms progressed, and he returned to the hospital the following day by ambulance. He was hypotensive with lactic acidosis and in acute renal failure. A blood film revealed infection with P. falciparum with a 39% parasitemia. He was started on IV quinidine and IV doxycycline. He responded well to treatment and his parasitemia dropped to 1%, but he remained in renal failure with acidosis. On the third day of hospitalization, he developed cardiac arrest. Resuscitation attempts were not successful, and he died on December 3.
Case 6. On August 5, a man aged 44 years returned to the United States after a 3-month business trip to Equatorial Guinea. He reported having malaria diagnosed three times while there, which he described as a mild cold. He also reported being started on atovaquone/proguanil chemoprophylaxis after one episode of malaria. He ran out of the medication just as he was leaving the country. He had onset of illness the day he returned and sought medical care on August 7. The physicians immediately suspected severe malaria and started empiric IV quinidine and IV doxycycline within 2 hours of his arrival in the emergency department. Initial laboratory findings indicated that he was acidotic and microscopy showed that he was infected with P. falciparum with an 18% parasitemia. Beginning the next morning, he developed rapidly progressive ARDS, renal failure, and refractory acidosis. He was intubated and required vasopressors for hypotension. His parasitemia had fallen to 6.5% after 24 hours of quinidine. On hospital day 3, he had a seizure and EEG indicated no cerebral activity. On August 9, he developed cardiac arrest and resuscitation was not successful.
Case 7. In early December, a man aged 55 years returned to the United States from a trip to Liberia to visit friends and relatives. A week later, he was found at home unresponsive and taken to the emergency department by ambulance. He had a history of untreated hypertension and diabetes and had apparently been feeling ill (with unspecified symptoms) for the week before admission. In the emergency department on December 9, he was found to be in diabetic ketoacidosis, hypotensive, febrile, and in renal failure. He was found to have E. coli bacteremia and treated with antibiotics, insulin, fluids, and vasopressors. On December 10, he also had P. falciparum diagnosed with a 0.6% parasitemia. Quinidine was initially unavailable and CDC was contacted for artesunate, which was sent. A supply of quinidine was found from a neighboring hospital 30 minutes later and started on December 10 at 5:30 a.m. Once the artesunate arrived on December 10 at 9:15 a.m., he was switched to IV artesunate. During the hospitalization, he developed gastrointestinal bleeding and remained hypotensive and progressively more acidotic, despite dialysis and repeated blood transfusions. Exploratory laparotomy revealed extensive necrotic bowel. This condition was deemed nonsurvivable, support was removed, and he died on December 12.
Case 8. In June, a man aged 33 years returned to the United States from a 5-week business trip to Liberia. He did not take any malaria chemoprophylaxis for fear of side effects. Two weeks after returning, he developed a fever. He sought medical care 3 days later. Malaria was diagnosed by microscopy in the emergency department, and he was given chloroquine and admitted to the hospital. The following morning, chloroquine was determined to be inappropriate because his infection was acquired in West Africa, and he was switched to atovaquone/proguanil. Later that day, the species was identified as P. falciparum with 30% parasitemia. Treatment was switched to IV quinidine and IV doxycycline and he was transferred to the ICU. On day 3 of hospitalization, he developed severe acute hemolytic anemia and renal failure. The following day he developed lactic acidosis and began having difficulty breathing; he was intubated and given an exchange transfusion. He was switched to IV artesunate, started on extracorporeal membrane oxygenation (ECMO), and transferred to another hospital. On hospital day 9, his respiratory function had improved and he was removed from ECMO. Immediately after the procedure, he had fixed and dilated pupils. A head CT revealed a massive cerebral hemorrhage; a craniotomy and clot excision were performed. The following day it was determined to be medically futile and life support measures were withdrawn. He died on hospital day 10.
Case 9. In July, a man aged 59 years from Sudan returned to the United States after a 3-month trip to Sudan to visit friends and relatives. On arrival into the United States, he experienced chills, sweats, and weakness; he did not seek medical attention. Approximately 3 weeks later on July 31, a family member found him at home confused and he was taken to the emergency department. He was diagnosed with severe malaria (parasitemia of 19%), renal failure, lactic acidosis, pancreatitis, and liver failure with severe jaundice. He was initially started on IV quinidine but developed QT-prolongation and supraventricular arrhythmias. CDC was contacted and artesunate was provided. His parasitemia dropped in response to the IV artesunate. During his hospitalization, he was also diagnosed with E. coli bacteremia, hepatitis C, and a pulmonary hemorrhage from a previously undiagnosed mass in his chest. He remained intubated and mechanically ventilated and on vasopressor support throughout his hospitalization. His liver failure, jaundice, blood pressure, and respiratory status continued to worsen despite maximal support. His family decided to forego further interventions, and he died on August 11.
Case 10. In July, a woman aged 34 years from the Republic of the Congo traveled to the United States for a family vacation. She began experiencing influenza-like symptoms 2 days before coming to the United States, but she did not seek care until 2 days after arriving. She went to the emergency department on July 16 where she was found to be thrombocytopenic, hypotensive, and tachycardic. A malaria blood film indicated she had severe P. falciparum infection with a 25% parasitemia. She was admitted to the ICU and started on IV quinidine and IV doxycycline. CDC was contacted and artesunate was provided in case she developed worsening hypotension on quinidine. The following day, she appeared to be improving with resolution of her hypotension and a parasitemia of 20%. That afternoon, she had a generalized seizure and became comatose. A head CT revealed brainstem herniation. The treating physicians switched her to IV artesunate. She remained comatose and was declared brain dead. She died on July 28.
Discussion
In 2013, a total of 1,727 malaria cases were reported in the United States, the third highest number recorded since 1970 and 10% lower than the highest number reported in 2011 during the preceding 40 years (25). An increasing trend in the number of cases has been observed since the early 1970s following the end of the Vietnam War. This pattern appears to be similar to that being reported in other parts of the world. For example, in the United Kingdom, the number of imported cases has increased substantially since the 1970s (30). In addition, in 2012, a total of 1,501 malaria cases were reported in the United Kingdom, a 9% increase from 2012 (n = 1,378) (31). The majority of the U.S. cases were acquired in sub-Saharan Africa, which is also similar to the data reported by the United Kingdom. Despite progress in reducing the number of malaria cases in regions where malaria is endemic (2), international travel appears to be growing steadily and use of appropriate prevention measures by travelers is still inadequate. The World Tourism Organization reported a total of 1.087 billion international travelers in 2013, with notable increases in overall travel (5%), travel to Asia and the Pacific (6%), and travel to Africa (6%) (26). Travel to North Africa continued to increase following declines that were a result of the Arab Spring and political transitions in North Africa in 2011. From 2012 to 2013, travel to Sub-Saharan Africa increased 5% (26).
International travelers are a heterogeneous group with different reasons for travel, levels of education, and potential barriers for chemoprophylaxis use. They represent both short-stay (e.g., air crew) and long-term travelers (e.g., Peace Corps volunteers, tourists, missionaries, disaster and relief workers, and military personnel) (32–35). In addition, reasons travelers do not use malaria chemoprophylaxis vary, including lack of awareness of their risks for malaria and the potential severity of the disease (32–35) and concerns about side effects of medications (35). As a result, interventions to improve chemoprophylaxis require a multifaceted approach. Health care providers need to tailor interventions to their particular populations to increase awareness, understanding, or acceptance of malaria chemoprophylaxis (36).
As international travel increases, prevention messages and health communication strategies become even more important for protecting the traveling community from communicable diseases. Prevention messages directed toward Africa-bound travelers, particularly those whose destination is West Africa, should be emphasized in early spring, accompanied with a reminder in late fall through early winter. Malaria prevention messages directed toward Asia-bound travelers, specifically those bound for India, should be intensified in late spring. Travelers should be informed of the risk for malaria and strongly encouraged to use protective measures, including chemoprophylaxis. Imported malaria can reintroduce malaria into regions, including the United States, where the disease is not endemic and environmental conditions are present that can support the lifecycle of the parasite, including the presence of a competent Anopheles vector.
Of the 1,720 imported cases, 242 (14%) reports did not provide information regarding U.S. resident status, 203 (12%) did not have information regarding travel history, and 286 (17%) did not have information on species. The percentage of cases with incomplete data elements did not change in 2013 compared with 2012, and it still remains unacceptably high. For most of the cases with missing residential status, travel history, and species, reporting to CDC was done electronically to NNDSS and not by the NMSS case report form. NNDSS is unable to receive malaria-specific data elements from state and local health departments. States and local health departments are strongly encouraged to report cases using the NMSS case report form until malaria-specific data can be received electronically by NNDSS. Importation status could not be determined for five cases because of lack of information. CDC provided diagnostic assistance with these five cases, but they were never reported to CDC by state/local health departments. Follow-up efforts did not yield the additional information necessary to classify them. Because incomplete reporting compromises efforts to examine trends in malaria cases in the United States and prevent infections among travelers, all elements on the NMSS case report form should be completed. Local and state health departments, health care providers, and other health personnel should be vigilant in reporting complete information for malaria cases. Specifically, if certain variables are not reported (e.g., species, residence, and country of acquisition), efforts should be made to obtain complete information for comprehensive analysis. Beginning in 2014, CSTE released a revised case definition for malaria highlighting the importance of determining the species and parasitemia at the time of diagnosis and strongly encouraging PCR testing for each case (37).
In the Caribbean region, endemic transmission of malaria ended in the mid-1960s, except on the island of Hispaniola, which includes the countries of the Dominican Republic and Haiti (38). An increase in the numbers of malaria cases acquired in Haiti had already been noted before the January 2010 earthquake (13). This increase continued throughout 2010 and was likely the result of both increased transmission in Haiti and increased volume of travel between the United States and Haiti by relief aid workers and Haitians returning to visit friends and relatives (23,39,40). The number of cases reported decreased from 172 in 2010 to 22 in 2013. Despite this significant decrease, the number of cases acquired in Haiti in 2013 is similar to the number acquired there before the 2010 earthquake (39). Of the 22 cases that were acquired in Haiti, only one patient reported taking prophylaxis. That patient reported taking chloroquine and mefloquine for prophylaxis, but it was not reported whether he had taken all of it. Failure to take chemoprophylaxis is the most common risk factor for acquisition of malaria among travelers to regions where the disease is endemic. Messages must be conveyed to VFR travelers that they are at substantial risk for malaria, despite beliefs that partial immunity offers protection from disease (41). Recent reports of emerging molecular markers of chloroquine drug resistance in Haiti (42,43) indicate a need for increased vigilance for evidence of clinical chloroquine chemoprophylaxis or treatment failure. However, chloroquine remains an effective choice for chemoprophylaxis and treatment of malaria acquired in Haiti. Health care providers should contact CDC to assist with the evaluation of possible chloroquine failures identified among U.S. travelers or Haitian immigrants to the United States. Efforts are underway to eliminate malaria from Central America and the Caribbean region by 2020; as a result, the number of cases from Haiti is expected to decrease over the next decade (44).
In 2013, one case of malaria was diagnosed in the United States in a traveler from Greece. Malaria was officially eliminated from Greece in 1974 (45,46). Since then, an average of 50 cases per year have been reported annually, the majority of which were imported from countries where malaria is endemic (45). In 2009, seven cases of malaria were diagnosed in Greece without a report of travel (47). That number increased to a high of 42 in 2011 (48). In response to these cases, the Hellenic Center for Disease Control and Prevention developed an action plan that included enhanced surveillance for malaria, updated treatment plans that included administration of antimalarials to immigrants from countries where malaria is endemic, provider and public education, and enhanced vector-control activities (48). The outbreak in Greece underscores the importance of investigating cases of malaria diagnosed in the United States, where no recent travel is indicated because of the potential for reintroduction of active transmission of malaria.
In 2013, a total of 14 cases were reported among military personnel, a 67% (n = 43) decrease from 2012, representing the lowest number of cases since 2003 (49). Approximately one third of the 2013 cases among military personnel were acquired in Afghanistan. Adherence among those military personnel who reported taking any chemoprophylaxis was 22% in 2012 and was 17% in 2013.This is substantially lower than the reported adherence by U.S. service members overall (range: 49%–100%) (50,51). Reasons for these differences might include overreporting of adherence among the survey respondents and missing information on chemoprophylaxis adherence reported to NMSS or AFHSC. Before 2010, cases among patients who were traveling for military duty to regions where malaria is endemic were only reported to CDC by local and state health departments and private health clinicians. However, since CDC partnered with AFHSC in 2010, additional cases occurring among the military are being identified that might have not been identified previously by local or state health departments or private health care providers, thus improving opportunities to monitor and survey trends or changes (e.g., in geographical transmission and prophylaxis or treatment failures among the deployed military population).
Of the 186 uncomplicated cases of P. vivax or P. ovale in men and in women who were not pregnant at the time of diagnosis (primaquine is contraindicated during pregnancy), only 75 (40%) received primaquine, the only antimalarial active against the dormant parasite liver forms and prevents relapses (52). In addition to their treatment for acute malaria, all persons who have mosquito-acquired P. vivax and P. ovale diagnosed and who are not G6PD deficient should receive a course of primaquine for relapse prevention (28). In 2011, the Food and Drug Administration reported that primaquine was back ordered because of manufacturing issues reported by Sanofi-Aventis, the only pharmaceutical company producing primaquine in the United States (53). Providers were urged to keep patients on weekly chloroquine prophylaxis to prevent relapses (http://www.cdc.gov/malaria/new_info/2011/primaquine.html). Only 28 (21%) of P. vivax and P. ovale patients who did not receive primaquine received chloroquine. Because the CDC surveillance report form does not record the dates of treatment, understanding and determining the intended purpose of chloroquine treatment in these cases is difficult. Primaquine became available again in late 2012 (54).
This report includes the second year of results of the molecular surveillance to assess the prevalence of antimalarial drug resistance markers. In 2013, CDC received 137 specimens from 29 state and local health departments. The prevalence of resistance markers varied for different drugs such as pyrimethamine, sulfadoxine, chloroquine, mefloquine, and atovaquone. No markers associated with resistance to artemisinin derivatives were identified. In many places around the world, malaria parasites have become resistant to the antimalarial drugs used to treat cases of malaria illness, and it is important to identify and track those resistance patterns. As CDC receives and tests more samples, it will be able to identify these evolving changes in resistance patterns in different countries; many countries do not conduct molecular surveillance and report it to publicly available databases. These data will help to formulate prevention and treatment recommendations for those traveling from the United States to areas where malaria is endemic and to determine country of acquisition for cases with no reported travel information. All cases of malaria diagnosed in the United States should be evaluated for drug resistance markers. This testing is available for all cases of malaria diagnosed in the United States and is conducted at CDC free of charge. CDC encourages all laboratories in the United States to use this service for all of the cases of malaria that they diagnose (http://www.cdc.gov/malaria/features/ars.html). Pretreatment blood collected for initial diagnostic tests (e.g., complete blood count) can be saved and sent to CDC for the drug resistance marker tests.
Ten fatal cases were reported in 2013, the highest number since 2001 (55). The fatalities were all from P. falciparum infections. One patient delayed seeking treatment after onset of symptoms, one was initially treated with oral antimalarials despite having a hyperparasitemia (≥5%), and one was discharged with empiric antibiotics without a recommended workup for fever in a traveler returning from an area where malaria is endemic. To facilitate a prompt diagnosis, providers also should include malaria in the differential diagnosis of fever in a person who has returned from travel to a malarious area. Signs and symptoms of malaria often are nonspecific but typically include fever. Other symptoms include headache, chills, increased sweating, back pain, myalgia, diarrhea, nausea, vomiting, and cough. Health care providers should ask all febrile patients for a travel history. Any delay in the diagnosis and treatment of malaria can result in complications, regardless of the effectiveness of the treatment regimen. Patients suspected of having malaria infection should be evaluated through microscopic examination of thick and thin blood films, adhering to Occupational Safety and Health Administration bloodborne pathogens standard (56). As a result of fear expressed during the Ebola outbreak in West Africa that began in 2014, CDC developed additional steps to inactivate viruses, including Ebola, during the slide preparation process (14). Thick blood films are more sensitive in detecting malaria parasites because the blood is more concentrated, which allows for a greater volume of blood to be examined. Thin films aid in parasite species identification and quantification (57). Blood films should be read immediately; off-hours, qualified personnel who can perform this function should be on-call. Laboratories unable to provide immediate blood film microscopy should maintain a supply of malaria antigen detection kits to assist with the initial diagnosis of malaria, which can subsequently be confirmed by microscopy or PCR.
The choice of a specific antimalarial treatment regimen should be made on the basis of several factors, including the probable geographic origin of the parasite, the Plasmodium species, parasite density, and the patient’s clinical status (58). A 2010 nationwide survey of laboratories in the United States indicated that most laboratories offered malaria diagnostic testing services but very few were in complete compliance with all of the Clinical and Laboratory Standards Institute guidelines for analysis and reporting of results (59). In addition, most laboratories reported very few cases annually (58). The case definition used in 2014 for malaria surveillance in the United States has been revised to include identification of malaria parasites, determination of species, and quantification of the parasitemia (37). Microscopy is still considered the best method for the immediate diagnosis of malaria; however, PCR testing is particularly valuable for species confirmation and should be used to confirm the results of microscopy and to evaluate for mixed infections. CDC’s Parasitology Diagnostic Service team provides no-cost diagnostic services to laboratories and health professionals diagnosing cases of malaria, including microscopy, PCR testing and drug resistance marker testing. Of the 1,727 cases, species confirmation was provided by CDC for 104 (6%) cases. A total of 137 specimens were submitted to CDC for drug resistance marker testing in 2013; for approximately half of those specimens, PCR testing identified or corrected the species. Increasing the proportion of cases with diagnosis confirmation and drug resistance marker testing will improve the understanding epidemiology of malaria diagnosed in the United States.
Patients with suspected or confirmed malaria who are severely ill should be treated aggressively with parenteral antimalarial therapy. Quinidine gluconate continues to be recommended for parenteral malaria therapy. However, this medication is no longer available in many hospital formularies. Because parenteral quinidine gluconate is potentially cardiotoxic, it should be administered in an intensive care setting with continuous cardiac and frequent blood pressure monitoring. As an alternative to quinidine gluconate, IV artesunate is also highly effective in the treatment of severe malaria and is available as an IND through CDC. Artesunate is stocked at nine sites around the United States and can be rapidly shipped at no cost to clinicians. Certain guidelines and eligibility requirements must be met to enroll a patient in the treatment protocol. Physicians who administer the drug to patients must notify CDC of any adverse event after administration and comply with the IND protocol (60). To enroll a patient with severe malaria in this treatment protocol, health care providers should call the CDC Malaria Hotline at 770-488-7788 or toll-free at 855-856-4713, Monday–Friday, 9 a.m.–5 p.m., Eastern time. At other times, callers should telephone 770-488-7100 and ask to speak with a CDC Malaria Branch clinician. Travelers and health care providers are encouraged to use CDC resources on malaria prevention and treatment (Table 9), and contact the CDC Malaria Branch for assistance with diagnostic or case management needs.
Detailed recommendations for preventing malaria are available to the general public 24 hours a day online at http://wwwnc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/malaria. Additional information on malaria prevention recommendations is also available through the online CDC malaria map application at http://www.cdc.gov/malaria/map. The application is an interactive map that provides information on malaria throughout the world. Users can search or browse countries, cities, and place names and get information about malaria in that particular location and see recommended malaria prophylaxis for that area. Also, CDC biannually publishes recommendations in Health Information for International Travel (commonly referred to as The Yellow Book), which is available and updated on the CDC Travelers’ Health website at http://www.cdc.gov/Features/TravelersHealth.html; the publication is also available for purchase from Oxford University Press, Inc., at https://global.oup.com/academic/?cc=us&lang=en& or telephone 1-800-451-7556 (Table 9).
Health care providers should be familiar with prevention, recognition, and treatment of malaria and are encouraged to consult appropriate sources for malaria prevention and treatment recommendations (Table 9). Health care providers should be aware of diagnostic and treatment resources available in their facilities, including availability at night or on weekends. A recent evaluation of malaria diagnosis capabilities among U.S. laboratories demonstrated that although malaria diagnostic testing services were available to the majority of U.S. laboratories surveyed, very few were in compliance with all of the current guidelines (35). To maintain and improve malaria and other parasitic disease diagnosis capabilities in the United States, CDC’s Parasitology Diagnostic Service team conducts training courses several times per year (http://www.cdc.gov/dpdx/index.html). Physicians seeking assistance with diagnosis (including telediagnosis) or treatment of patients with suspected or confirmed malaria should call CDC’s Malaria Hotline at telephone 770-488-7788 or toll-free 855-856-4713 during regular business hours or CDC’s Emergency Operations Center at telephone 770-488-7100 during evenings, weekends, and holidays (ask to page the person on call for the Malaria Branch), or access CDC’s Internet site at http://www.cdc.gov/malaria/diagnosis_treatment/index.html. These resources are intended for use by health care providers only.
Acknowledgments
The authors acknowledge the state, territorial, and local health departments; health care providers; laboratories; and the AFHSC for providing this information to CDC. Laboratory support was provided by the malaria diagnostic reference laboratory and the support of Luciana Silva-Flannery, Curtis Huber, Dragan Ljolje, Venkatachalam Udhayakumar and John Barnwell in obtaining molecular surveillance data. Molecular surveillance work was supported by funds from CDC Antimicrobial Resistance Working Group.
Corresponding author: Paul M. Arguin, Malaria Branch, Division of Parasitic Diseases and Malaria, Center for Global Health. Telephone: 404-718-4703; E-mail: pma0@cdc.gov.
References
- World Health Organization. World malaria report 2014. Geneva, Switzerland: WHO Press; 2014.
- World Health Organization. World malaria report 2013. Geneva, Switzerland: WHO Press; 2013.
- Pan American Health Organization. Report for registration of malaria eradication from United States of America. Washington, DC: Pan American Health Organization; 1969.
- Andrews JM, Quinby GE, Langmuir AD. Malaria eradication in the United States. Am J Public Health Nations Health 1950;40:1405–11 . CrossRef
- Mckay T, Bianco T, Rhodes L, Barnett S. Prevalence of Dirofilaria immitis (Nematoda: Filarioidea) in mosquitoes from northeast Arkansas, the United States. J Med Entomol 2013;50:871–8. CrossRef
- Cullen KA, Arguin PM. Malaria surveillance—United States, 2011. MMWR Surveill Summ 2013;62(No. SS-5).
- CDC. Local transmission of Plasmodium vivax malaria—Palm Beach County, Florida, 2003. MMWR Morb Mortal Wkly Rep 2003;52:908–11.
- Leder K, Black J, O’Brien D, et al. Malaria in travelers: a review of the GeoSentinel surveillance network. Clin Infect Dis 2004;39:1104–12 . CrossRef
- CDC. National notifiable disease surveillance system. Atlanta, GA: US Department of Health and Human Services, CDC; 2012. http://wwwn.cdc.gov/nndss/default.aspx
- Council of State and Territorial Epidemiologists. Public health reporting and national notification for malaria. Atlanta, GA; 2009. http://c.ymcdn.com/sites/www.cste.org/resource/resmgr/PS/09-ID-47.pdf
- World Health Organization. Terminology of malaria and of malaria eradication: report of a drafting committee. Geneva, Switzerland: World Health Organization; 1963.
- World Health Organization. Management of severe malaria: a practical handbook. Third ed. Geneva, Switzerland: WHO Press; 2012.
- Mali S, Tan KR, Arguin PM. Malaria surveillance—United States, 2009. MMWR Surveill Summ 2011;60(No. SS-3).
- CDC. Guidance for malaria diagnosis in patients suspected of Ebola infection in the United States. Atlanta, GA: US Department of Health and Human Services; CDC, 2014. http://www.cdc.gov/malaria/new_info/2014/malaria_ebola.htm
- BinaxNOW Malaria [package insert]. Scarborough, Maine: Inverness Medical Professional Diagnostics; 2007.
- CDC. Malaria rapid diagnostic test. MMWR Morb Mortal Wkly Rep 2007;56:686.
- Bacon DJ, McCollum AM, Griffing SM, et al. Dynamics of malaria drug resistance patterns in the Amazon basin region following changes in Peruvian national treatment policy for uncomplicated malaria. Antimicrob Agents Chemother 2009;53:2042–51 . CrossRef
- Korsinczky M, Chen N, Kotecka B, Saul A, Rieckmann K, Cheng Q. Mutations in Plasmodium falciparum cytochrome b that are associated with atovaquone resistance are located at a putative drug-binding site. Antimicrob Agents Chemother 2000;44:2100–8 . CrossRef
- Price RN, Uhlemann AC, Brockman A, et al. Mefloquine resistance in Plasmodium falciparum and increased pfmdr1 gene copy number. Lancet 2004;364:438–47 . CrossRef
- Takala-Harrison S, Clark TG, Jacob CG, et al. Genetic loci associated with delayed clearance of Plasmodium falciparum following artemisinin treatment in Southeast Asia. Proceedings of the National Academy of Sciences 2012;110(240–5).
- Ariey F, Witkowski B, Amaratunga C, et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 2014;505:50–5 . CrossRef
- Talundzic E, Okoth SA, Congpuong K, et al. Selection and spread of artemisinin-resistant alleles in Thailand prior to the global artemisinin resistance containment campaign. PLoS Pathog 2015;11:e1004789 . CrossRef
- CDC. Malaria surveillance—United States, 2010. MMWR Surveill Summ 2012;61(No. SS-2).
- CDC. Malaria surveillance—United States, 2012. MMWR Surveill Summ 2014;66(No. SS-12).
- Mali S, Steele S, Slustker L, Arguin PM. Malaria surveillance—United States, 2008. MMWR Surveill Summ 2010;59(No. SS-7).
- World Tourism Organization. UNWTO Tourism Highlights, 2013 Edition. Madrid: UNWTO Publications; 2013. http://www.e-unwto.org/content/q27534/fulltext.pdf
- Armed Forces Health Surveillance Center (AFHSC). Update: malaria, US Armed Forces, 2012. MSMR 2013;20:2–5, discussion 5.
- CDC. Guidelines for treatment of malaria in the United States. July 1, 2013. Atlanta, GA: US Department of Health and Human Services, CDC; 2012. http://www.cdc.gov/malaria/resources/pdf/treatmenttable.pdf
- Plucinski MM, Huber CS, Akinyi S, et al. Novel mutation in cytochrome b of Plasmodium falciparum in one of two atovaquone-proguanil treatment failures in travelers returning from same site in Nigeria. Open Forum Infect Dis 2014;1:ofu059 . CrossRef
- Dobson M. The history of malaria in England. Wellcome Trust; 1999. http://malaria.wellcome.ac.uk/doc_wtd023991.html
- Public Health England. Malaria imported into the United Kingdom in 2013: implications for those advising travellers. London, England. 2014. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/326927/hpr1614.pdf
- Baggett HC, Graham S, Kozarsky PE, et al. Pretravel health preparation among US residents traveling to India to VFRs: importance of ethnicity in defining VFRs. J Travel Med 2009;16:112–8 . CrossRef
- Balaban V, Warnock E, Ramana Dhara V, Jean-Louis LA, Sotir MJ, Kozarsky P. Health risks, travel preparation, and illness among public health professionals during international travel. Travel Med Infect Dis 2014;12:349–54 . CrossRef
- Landman KZ, Tan KR, Arguin PM. Knowledge, attitudes, and practices regarding antimalarial chemoprophylaxis in U.S. Peace Corps Volunteers—Africa, 2013. MMWR Morb Mortal Wkly Rep 2014;63:516–7.
- Selent M, de Rochars VMB, Stanek D, et al. Malaria prevention knowledge, attitudes, and practices (KAP) among international flying pilots and flight attendants of a US commercial airline. J Travel Med 2012;19:366–72 . CrossRef
- Diara M, Nowosiwsky A, Harmen S, Burke N, Alilio M. Enabling factors for improved malaria chemoprophylaxis compliance. Am J Trop Med Hyg 2012;87:960–1 . CrossRef
- Council of State and Territorial Epidemiologists. Public health reporting and national notification for malaria. Atlanta, GA. http://c.ymcdn.com/sites/www.cste.org/resource/resmgr/PS/13-ID-08.pdf
- Pan American Health Organization. Status of malaria eradication in the Americas, 18th report. PAHO CSP 18/7. Washington, DC: Pan American Health Organization; 1970. http://new.paho.org/hq/index.php?lang=en
- Agarwal A, McMorrow M, Arguin PM. The increase of imported malaria acquired in Haiti among US travelers in 2010. Am J Trop Med Hyg 2012;86:9–10 . CrossRef
- Townes D, Existe A, Boncy J, et al. Malaria survey in post-earthquake Haiti—2010. Am J Trop Med Hyg 2012;86:29–31 . CrossRef
- CDC. CDC health information for international travel 2012. Chapter: Immigrants returning home to visit friends and relatives (VFRs) by Jay Keystone. New York: Oxford University Press; 2012. 547–51.
- Londono BL, Eisele TP, Keating J, et al. Chloroquine-resistant haplotype Plasmodium falciparum parasites, Haiti. Emerg Infect Dis 2009;15:735–40 . CrossRef
- Londono-Renteria B, Eisele TP, Keating J, Bennett A, Krogstad DJ. Genetic diversity in the merozoite surface protein 1 and 2 genes of Plasmodium falciparum from the Artibonite Valley of Haiti. Acta Trop 2012;121:6–12 . CrossRef
- The Global Fund. Ten countries rally to eliminate malaria in Central America and the Caribbean. 2013. Geneva, Switzerland. http://www.theglobalfund.org/en/mediacenter/newsreleases/2013-06-28_Ten_Countries_Rally_to_Eliminate_Malaria_in_Central_America_and_the_Caribbean/
- Danis K, Baka A, Lenglet A, et al. Autochthonous Plasmodium vivax malaria in Greece, 2011. Euro Surveill 2011;16:1–5.
- Vakali A, Patsoula E, Spanakos G, et al. Malaria in Greece, 1975 to 2010. Euro Surveill 2012;17:20322.
- Hellenic Center for Disease Control and Prevention. Epidemiological surveillance report on malaria in Greece, up to 11/16/2013. http://www.keelpno.gr
- Hellenic Center for Disease Control and Prevention. Epidemiological surveillance report on malaria in Greece, 2014. http://www.keelpno.gr.
- Armed Forces Health Surveillance Center (AFHSC). Update: malaria, US armed forces, 2013. MSMR 2014;21:4–7.
- Armed Forces Health Surveillance Center. Surveillance snapshot: Self-reported malaria prophylaxis compliance among US service members with diagnosed malaria, 2008–2013. MSMR 2014;21:15.
- Brisson M, Brisson P. Compliance with antimalaria chemoprophylaxis in a combat zone. Am J Trop Med Hyg 2012;86:587–90 . CrossRef
- Griffith KS, Lewis LS, Mali S, Parise ME. Treatment of malaria in the United States: a systematic review. JAMA 2007;297:2264–77 . CrossRef
- CDC. Primaquine shortage. 2011. http://www.cdc.gov/malaria/new_info/2011/primaquine.html
- CDC. Primaquine now available. 2012. http://www.cdc.gov/malaria/new_info/2012/primaquine.html
- Filler S, Causer LM, Newman RD, et al. Malaria surveillance—United States, 2001. MMWR Surveill Summ 2003;52(No. SS-5).
- CDC. Malaria diagnosis & treatment in the United States. 2012. http://www.cdc.gov/malaria/diagnosis_treatment/index.html
- CDC. Malaria diagnosis (United States). Atlanta, GA: US Department of Health and Human Services, CDC; 2012. http://www.cdc.gov/malaria/diagnosis_treatment/diagnosis.html.
- Baird JK. Effectiveness of antimalarial drugs. N Engl J Med 2005;352:1565–77 . CrossRef
- Abanyie FA, Arguin PM, Gutman J. State of malaria diagnostic testing at clinical laboratories in the United States, 2010: a nationwide survey. Malar J 2011;10:340 . CrossRef
- CDC. New medication for severe malaria available under an investigational new drug protocol. MMWR Morb Mortal Wkly Rep 2007;56:769–73.
* The term United States includes all states and territories.
† Countries that are considered West Africa include Benin, Burkina Faso, Cape Verde, Cóte d’Ivoire, Gambia, Ghana, Guinea, Guinea-Bissau, Liberia, Mali, Mauritania, Niger, Nigeria, Senegal, Sierra Leone, Togo, and West Africa, unspecified.
§ Countries that are considered South Asia include Afghanistan, Bangladesh, Bhutan, India, Maldives, Nepal, Pakistan, and Sri Lanka (Ceylon).
 FIGURE 1. Number of malaria cases among U.S. military personnel and U.S. and foreign civilians — United States, 1973–2013*
FIGURE 1. Number of malaria cases among U.S. military personnel and U.S. and foreign civilians — United States, 1973–2013*
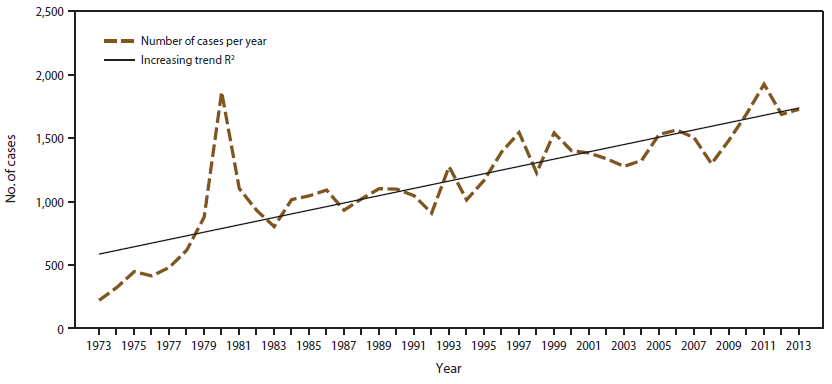
*R2 = 0.6857 is the average rate in rise of cases over time.
 TABLE 1. Number of malaria cases* among U.S. military personnel and U.S. and foreign civilians — United States, 1970–2013
TABLE 1. Number of malaria cases* among U.S. military personnel and U.S. and foreign civilians — United States, 1970–2013
| Year | U.S. military personnel | U.S. civilians | Foreign residents | Status not recorded | Total |
|---|---|---|---|---|---|
| 1970 | 4,096 | 90 | 44 | 17 | 4,247 |
| 1971 | 2,975 | 79 | 69 | 57 | 3,180 |
| 1972 | 454 | 106 | 54 | 0 | 614 |
| 1973 | 41 | 103 | 78 | 0 | 222 |
| 1974 | 21 | 158 | 144 | 0 | 323 |
| 1975 | 17 | 199 | 232 | 0 | 448 |
| 1976 | 5 | 178 | 227 | 5 | 415 |
| 1977 | 11 | 233 | 237 | 0 | 481 |
| 1978 | 31 | 270 | 315 | 0 | 616 |
| 1979 | 11 | 229 | 634 | 3 | 877 |
| 1980 | 26 | 303 | 1,534 | 1 | 1,864 |
| 1981 | 21 | 273 | 809 | 0 | 1,103 |
| 1982 | 8 | 348 | 574 | 0 | 930 |
| 1983 | 10 | 325 | 468 | 0 | 803 |
| 1984 | 24 | 360 | 632 | 0 | 1,016 |
| 1985 | 31 | 446 | 568 | 0 | 1,045 |
| 1986 | 35 | 410 | 646 | 0 | 1,091 |
| 1987 | 23 | 421 | 488 | 0 | 932 |
| 1988 | 33 | 550 | 440 | 0 | 1,023 |
| 1989 | 35 | 591 | 476 | 0 | 1,102 |
| 1990 | 36 | 558 | 504 | 0 | 1,098 |
| 1991 | 22 | 585 | 439 | 0 | 1,046 |
| 1992 | 29 | 394 | 481 | 6 | 910 |
| 1993 | 278 | 519 | 453 | 25 | 1,275 |
| 1994 | 38 | 524 | 370 | 82 | 1,014 |
| 1995 | 12 | 599 | 461 | 95 | 1,167 |
| 1996 | 32 | 618 | 636 | 106 | 1,392 |
| 1997 | 28 | 698 | 592 | 226 | 1,544 |
| 1998 | 22 | 636 | 361 | 208 | 1,227 |
| 1999 | 55 | 833 | 381 | 271 | 1,540 |
| 2000 | 46 | 827 | 354 | 175 | 1,402 |
| 2001 | 18 | 891 | 316 | 158 | 1,383 |
| 2002 | 33 | 849 | 272 | 183 | 1,337 |
| 2003 | 36 | 767 | 306 | 169 | 1,278 |
| 2004 | 32 | 775 | 282 | 235 | 1,324 |
| 2005 | 36 | 870 | 297 | 325 | 1,528 |
| 2006 | 50 | 736 | 217 | 561 | 1,564 |
| 2007 | 33 | 701 | 263 | 508 | 1,505 |
| 2008 | 19 | 510 | 176 | 593 | 1,298 |
| 2009 | 18 | 661 | 201 | 604 | 1,484 |
| 2010 | 46 | 1,085 | 368 | 192 | 1,691 |
| 2011 | 91 | 1,098 | 386 | 350 | 1,925 |
| 2012 | 43 | 1,121 | 328 | 195 | 1,687 |
| 2013 | 14 | 1,123 | 348 | 242 | 1,727 |
*A case was defined as symptomatic or asymptomatic illness that occurs in the United States or one of its territories in a person who has laboratory-confirmed malaria parasitemia (microscopy or PCR), regardless of whether the person had previous attacks of malaria while in other countries. A subsequent attack of malaria occurring in a person is counted as an additional case if the demonstrated Plasmodium species differs from the initially identified species or if it is indicated as a relapsing infection demonstrating the same Plasmodium species as identified previously. If a subsequent attack of malaria occurring as a result of a drug resistance failure then the case is not counted as an additional case.
 TABLE 2. Number of malaria cases, by Plasmodium species and year — United States, 2009–2013
TABLE 2. Number of malaria cases, by Plasmodium species and year — United States, 2009–2013
| Plasmodium species | 2009 | 2010 | 2011 | 2012 | 2013 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | (%) | No. | (%) | No. | (%) | No. | (%) | No. | (%) | |
| P. falciparum | 687 | (46.3) | 982 | (58.1) | 948 | (49.3) | 985 | (58.4) | 1,051 | (60.8) |
| P. vivax | 166 | (11.2) | 325 | (19.2) | 420 | (21.8) | 280 | (16.6) | 243 | (14.1) |
| P. malariae | 32 | (2.1) | 35 | (2.1) | 50 | (2.6) | 54 | (3.2) | 43 | (2.5) |
| P. ovale | 29 | (2.0) | 33 | (1.9) | 51 | (2.6) | 59 | (3.5) | 64 | (3.7) |
| P. knowlesi | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| Mixed | 13 | (0.9) | 13 | (0.8) | 21 | (1.1) | 21 | (1.2) | 40 | (2.3) |
| Undetermined | 557 | (37.5) | 303 | (17.9) | 435 | (22.6) | 288 | (17.1) | 286 | (16.6) |
| Total | 1,484 | (100) | 1,691 | (100) | 1,925 | (100) | 1,687 | (100) | 1,727 | (100) |
 TABLE 3. Number of imported malaria cases, by country of acquisition and Plasmodium species — United States, 2013
TABLE 3. Number of imported malaria cases, by country of acquisition and Plasmodium species — United States, 2013
| Country of acquisition | P. vivax | P. falciparum | P. malariae | P. ovale | Mixed | Unknown | Total |
|---|---|---|---|---|---|---|---|
| Africa | 52 | 944 | 32 | 56 | 27 | 139 | 1,250 |
| Angola | 0 | 9 | 0 | 0 | 0 | 0 | 9 |
| Benin | 1 | 10 | 1 | 1 | 0 | 1 | 14 |
| Burkina Faso | 1 | 14 | 0 | 0 | 0 | 1 | 16 |
| Burundi | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Cameroon | 2 | 51 | 2 | 1 | 0 | 7 | 63 |
| Central African Republic | 1 | 2 | 0 | 0 | 0 | 1 | 4 |
| Chad | 0 | 6 | 0 | 0 | 2 | 1 | 9 |
| Congo, Republic of | 0 | 20 | 1 | 1 | 1 | 0 | 23 |
| Cóte d’Ivoire | 0 | 30 | 1 | 5 | 0 | 6 | 42 |
| Democratic Republic of Congo | 0 | 4 | 0 | 0 | 0 | 1 | 5 |
| Equatorial Guinea | 0 | 6 | 0 | 1 | 0 | 0 | 7 |
| Eritrea | 4 | 1 | 0 | 0 | 0 | 2 | 7 |
| Ethiopia | 17 | 6 | 0 | 1 | 1 | 4 | 29 |
| Gabon | 0 | 3 | 0 | 0 | 0 | 0 | 3 |
| Gambia | 1 | 12 | 0 | 0 | 0 | 0 | 13 |
| Ghana | 3 | 84 | 4 | 3 | 4 | 8 | 106 |
| Guinea | 0 | 39 | 1 | 0 | 3 | 3 | 46 |
| Kenya | 2 | 31 | 3 | 3 | 1 | 6 | 46 |
| Liberia | 1 | 102 | 6 | 5 | 1 | 15 | 130 |
| Malawi | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Mali | 0 | 18 | 1 | 1 | 0 | 4 | 24 |
| Mauritania | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Mozambique | 0 | 4 | 0 | 1 | 0 | 0 | 5 |
| Namibia | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Niger | 0 | 2 | 0 | 0 | 0 | 0 | 2 |
| Nigeria | 6 | 210 | 3 | 11 | 7 | 28 | 265 |
| Senegal | 0 | 7 | 0 | 1 | 0 | 4 | 12 |
| Sierra Leone | 0 | 103 | 3 | 2 | 2 | 17 | 127 |
| Somalia | 1 | 2 | 0 | 0 | 0 | 0 | 3 |
| South Africa | 0 | 3 | 0 | 1 | 0 | 0 | 4 |
| South Sudan | 0 | 8 | 0 | 0 | 0 | 1 | 9 |
| Sudan | 3 | 32 | 0 | 1 | 1 | 4 | 41 |
| Tanzania | 1 | 13 | 1 | 2 | 0 | 1 | 18 |
| Togo | 0 | 15 | 0 | 0 | 0 | 1 | 16 |
| Uganda | 3 | 41 | 0 | 8 | 1 | 11 | 64 |
| Zambia | 0 | 4 | 0 | 0 | 0 | 0 | 4 |
| Zimbabwe | 0 | 2 | 0 | 0 | 0 | 0 | 2 |
| Central Africa, unspecified | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| East Africa, unspecified | 3 | 8 | 1 | 2 | 0 | 3 | 17 |
| Southern Africa, unspecified | 0 | 2 | 0 | 0 | 0 | 0 | 2 |
| West Africa, unspecified | 0 | 9 | 1 | 0 | 3 | 5 | 18 |
| Africa, unspecified | 1 | 29 | 3 | 5 | 0 | 3 | 41 |
| Asia | 119 | 13 | 6 | 3 | 7 | 16 | 164 |
| Afghanistan | 5 | 0 | 0 | 1 | 0 | 0 | 6 |
| Bangladesh | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Burma (Myanmar) | 3 | 0 | 0 | 0 | 0 | 0 | 3 |
| Cambodia | 2 | 0 | 0 | 0 | 0 | 0 | 2 |
| India | 79 | 11 | 6 | 1 | 7 | 11 | 115 |
| Pakistan | 20 | 1 | 0 | 1 | 0 | 3 | 25 |
| South Korea | 2 | 0 | 0 | 0 | 0 | 0 | 2 |
| Thailand | 6 | 0 | 0 | 0 | 0 | 2 | 8 |
| Southeast Asia, unspecified | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Asia, unspecified | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Central America and the Caribbean | 8 | 27 | 1 | 1 | 0 | 4 | 41 |
| Belize | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Dominican Republic | 0 | 4 | 0 | 0 | 0 | 0 | 4 |
| El Salvador | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Guatemala | 2 | 0 | 0 | 1 | 0 | 0 | 3 |
| Haiti | 0 | 22 | 0 | 0 | 0 | 0 | 22 |
| Honduras | 4 | 0 | 0 | 0 | 0 | 1 | 5 |
| Mexico | 1 | 0 | 0 | 0 | 0 | 1 | 2 |
| Nicaragua | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Panama | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Central America, unspecified | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| South America | 30 | 11 | 0 | 0 | 4 | 8 | 53 |
| Argentina | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Brazil | 2 | 0 | 0 | 0 | 0 | 1 | 3 |
| Ecuador | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Guyana | 20 | 7 | 0 | 0 | 4 | 6 | 37 |
| Peru | 8 | 2 | 0 | 0 | 0 | 0 | 10 |
| South America, unspecified | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Oceania | 6 | 1 | 0 | 0 | 0 | 1 | 8 |
| Papua New Guinea | 4 | 1 | 0 | 0 | 0 | 1 | 6 |
| Solomon Islands | 2 | 0 | 0 | 0 | 0 | 0 | 2 |
| Europe | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Greece | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Unknown | 24 | 51 | 4 | 4 | 2 | 118 | 203 |
| Total | 240 | 1,047 | 43 | 64 | 40 | 286 | 1,720 |
 FIGURE 2. Number* of malaria cases, by state in which the disease was diagnosed — United States, 2013
FIGURE 2. Number* of malaria cases, by state in which the disease was diagnosed — United States, 2013
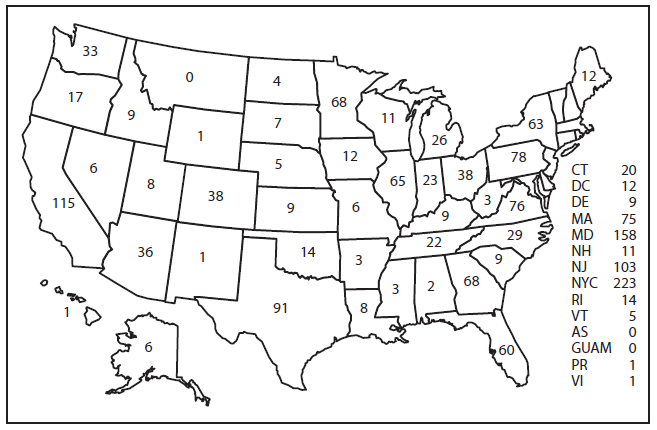
*N = 1,727.
 TABLE 4. Number and percentage of imported malaria cases among U.S. and foreign residents, by region of acquisition — United States, 2013
TABLE 4. Number and percentage of imported malaria cases among U.S. and foreign residents, by region of acquisition — United States, 2013
| Area or region | United States | Foreign | Total | |||
|---|---|---|---|---|---|---|
| No. | (%) | No. | (%) | No. | (%) | |
| Africa | 941 | (82.8) | 247 | (71.0) | 1,188 | (80.0) |
| Asia | 89 | (7.8) | 67 | (19.3) | 156 | (10.5) |
| South America | 38 | (3.3) | 12 | (3.4) | 50 | (3.4) |
| Central America/ Caribbean | 32 | (2.8) | 6 | (1.7) | 38 | (2.6) |
| Oceania | 8 | (0.7) | 0 | (0) | 8 | (0.5) |
| Europe | 0 | (0) | 1 | (0.3) | 1 | (0.1) |
| Unknown | 28 | (2.4) | 15 | (4.3) | 43 | (2.9) |
| Total | 1,136 | (100) | 348 | (100) | 1,484 | (100) |
 TABLE 5. Number and percentage of imported malaria cases, by interval between date of arrival in the United States and onset of illness and Plasmodium species* — United States, 2013
TABLE 5. Number and percentage of imported malaria cases, by interval between date of arrival in the United States and onset of illness and Plasmodium species* — United States, 2013
| Interval (days) | P. vivax | P. falciparum | P. malariae | P. ovale | Mixed | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | (%) | No. | (%) | No. | (%) | No. | (%) | No. | (%) | No. | (%) | |
| <0† | 10 | (7.6) | 105 | (13.7) | 1 | (4.5) | 1 | (3.0) | 3 | (11.5) | 120 | (12.2) |
| 0–29 | 60 | (45.4) | 620 | (80.8) | 12 | (54.5) | 14 | (42.4) | 17 | (65.4) | 723 | (73.8) |
| 30–89 | 24 | (18.2) | 34 | (4.4) | 7 | (31.8) | 12 | (36.4) | 5 | (19.2) | 82 | (8.4) |
| 90–179 | 19 | (14.4) | 5 | (0.7) | 1 | (4.5) | 3 | (9.1) | 1 | (3.8) | 29 | (3.0) |
| 180–364 | 19 | (14.4) | 1 | (0.1) | 0 | (0) | 2 | (6.1) | 0 | (0) | 22 | (2.2) |
| ≥365 | 0 | (0) | 2 | (0.3) | 1 | (4.5) | 1 | (3.0) | 0 | (0) | 4 | (0.4) |
| Total | 133 | (100.0) | 766 | (100.0) | 22 | (100.0) | 33 | (100.0) | 26 | (100.0) | 980 | (100.0) |
*Data are not included for persons for whom Plasmodium species, date of arrival in the United States, or date of onset of illness is unknown.
† Cases in this row are in patients who had onset of illness before arriving in the United States.
 TABLE 6. Number* and percentage of imported malaria cases among U.S. civilians, by purpose of travel at the time of acquisition — United States, 2013
TABLE 6. Number* and percentage of imported malaria cases among U.S. civilians, by purpose of travel at the time of acquisition — United States, 2013
| Category | No. | (%) |
|---|---|---|
| Visiting friends and relatives | 635 | (56.6) |
| Tourist | 43 | (3.8) |
| Missionary or dependent | 69 | (6.2) |
| Business representative | 92 | (8.2) |
| Student or teacher | 32 | (2.9) |
| Air crew or sailor | 0 | (0) |
| Peace Corps | 7 | (0.6) |
| Other | 26 | (2.3) |
| Unknown | 218 | (19.4) |
*N = 1,122
 TABLE 7. Comparison of malaria species reported on specimen submission form and polymerase chain reaction results* — United States, 2013
TABLE 7. Comparison of malaria species reported on specimen submission form and polymerase chain reaction results* — United States, 2013
| Species reported on specimen submission form | Species identified by PCR | |||||
|---|---|---|---|---|---|---|
| P. falciparum | P. vivax | P. ovale | P. malariae | Mixed | Total | |
| P. falciparum | 42 | 0 | 1 | 0 | 0 | 43 |
| P. vivax | 0 | 7 | 1 | 0 | 0 | 8 |
| P. ovale | 2 | 1 | 2 | 0 | 0 | 5 |
| P. malariae | 2 | 0 | 0 | 0 | 0 | 2 |
| Mixed | 0 | 0 | 1 | 2 | 1 | 4 |
| Unknown/Missing | 54? | 7 | 9 | 4 | 1 | 75 |
| Total | 100 | 15 | 14 | 6 | 2 | 137 |
*Of the 137 samples CDC tested for genetic markers associated with resistance to antimalarial drugs, 85 (62%) were found to have incorrect species annotated on the specimen submission form.
† Includes one sample reported as Babesia.
 TABLE 8. Antimalarial drug resistance test* results among Plasmodium falciparum specimens, by drug and region of malaria acquisition — United States, 2013
TABLE 8. Antimalarial drug resistance test* results among Plasmodium falciparum specimens, by drug and region of malaria acquisition — United States, 2013
| Resistance test | Region | |||||||
|---|---|---|---|---|---|---|---|---|
| Africa | Asia | Central American and the Caribbean | South America | Oceana | Middle East | Unknown | Total | |
| Pyrimethamine (No.) | 74 | 0 | 1 | 1 | 0 | 0 | 19 | 95 |
| Sensitive (%) | (5) | (0) | (100) | (0) | (0) | (0) | (5) | (6) |
| 1 resistance marker (%) | (0) | (0) | (0) | (0) | (0) | (0) | (0) | (0) |
| 2 resistance markers (%) | (8) | (0) | (0) | (0) | (0) | (0) | (0) | (6) |
| 3 or more resistance markers (%) | (86) | (0) | (0) | (100) | (0) | (0) | (95) | (87) |
| Sulfadoxine (No.) | 76 | 0 | 1 | 1 | 0 | 0 | 20 | 98 |
| Sensitive (%) | (26) | (0) | (0) | (0) | (0) | (0) | (20) | (24) |
| 1 resistance marker (%) | (36) | (0) | (100) | (0) | (0) | (0) | (45) | (38) |
| 2 resistance markers (%) | (24) | (0) | (0) | (100) | (0) | (0) | (15) | (22) |
| 3 or more resistance markers (%) | (14) | (0) | (0) | (0) | (0) | (0) | (20) | (15) |
| Chloroquine (No.) | 77 | 0 | 1 | 1 | 0 | 0 | 20 | 99 |
| Sensitive (%) | (48) | (0) | (100) | (0) | (0) | (0) | (40) | (46) |
| 1 resistance marker (%) | (52) | (0) | (0) | (100) | (0) | (0) | (60) | (54) |
| Mefloquine (No.) | 72 | 0 | 0 | 1 | 0 | 0 | 16 | 89 |
| Sensitive (%) | (100) | (0) | (0) | (100) | (0) | (0) | (100) | (100) |
| 1 resistance marker (%) | (0) | (0) | (0) | (0) | (0) | (0) | (0) | (0) |
| Atovaquone (No.) | 75 | 0 | 1 | 1 | 0 | 0 | 17 | 94 |
| Sensitive (%) | (99) | (0) | (100) | (100) | (0) | (0) | (100) | (99) |
| 1 resistance marker (%) | (1) | (0) | (0) | (0) | (0) | (0) | (0) | (1) |
| Artemisinin (No.) | 60 | 0 | 1 | 1 | 0 | 0 | 14 | 76 |
| Sensitive (%) | (100) | (100) | (100) | (100) | (100) | (100) | (100) | (100) |
| 1 resistance marker (%) | (0) | (0) | (0) | (0) | (0) | (0) | (0) | (0) |
* Sensitive is defined as having no resistance markers. For chloroquine, mefloquine, atovaquone, and artemisinin testing, resistance is defined as having any resistance markers. For pyrimethamine and sulfadoxine testing, resistance is defined by the number of resistance markers identified (i.e., low [one mutation], moderate [two mutations], or high [three or more mutations]).
 TABLE 9. Sources for malaria prophylaxis, diagnosis, and treatment recommendations
TABLE 9. Sources for malaria prophylaxis, diagnosis, and treatment recommendations
| Type of information | Source | Availability | Telephone number, internet address, or electronic mail address |
|---|---|---|---|
| Prophylaxis | CDC’s Traveler’s Health Internet site (includes online access to Health Information for International Travel) | 24 hours/day | http://wwwnc.cdc.gov/travel |
| Health Information for International Travel (The Yellow Book) | Order from Oxford University Press, Inc. Order Fulfillment 198 Madison Avenue, New York, NY 10016-4314 |
800-451-7556 or http://www.oup.com/us/ | |
| CDC’s Malaria Branch Internet site with Malaria Information and Prophylaxis, By Country (Red Pages) | 24 hours/day | http://www.cdc.gov/malaria/travelers/country_table/a.html | |
| CDC Malaria Map Application | 24 hours/day | http://www.cdc.gov/malaria/map | |
| Diagnosis | CDC’s Division of Parasitic Diseases and Malaria diagnostic internet site (DPDx) | 24 hours/day | http://www.dpd.cdc.gov/dpdx |
| CDC’s Division of Parasitic Diseases and Malaria diagnostic CD-ROM (DPDx) | Order by electronic mail from CDC Division of Parasitic Diseases and Malaria | dpdx@cdc.gov | |
| Treatment | CDC Malaria Branch | 9:00 am–5:00 pm Eastern time, Monday–Friday | 770-488-7788 or toll-free 855-856-4713* |
| CDC Malaria Branch | 5:00 pm–9:00 am Eastern time on weekdays and all day weekends and holidays | 770-488-7100* (This number is for the CDC’s Emergency Operations Center. Ask staff member to page the person on call for the Malaria Branch.) http://www.cdc.gov/malaria/diagnosis_treatment/treatment.html |
*These numbers are meant for use by health care professionals only.
Suggested citation for this article: Cullen KA, Mace KE, Arguin PM. Malaria Surveillance — United States, 2013. MMWR Surveill Summ 2016;65(No. SS-2)(No. SS-2):1–22. DOI: http://dx.doi.org/10.15585/mmwr.ss6502a1.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (https://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.
- Page last reviewed: June 21, 2017
- Page last updated: June 21, 2017
- Content source:


 ShareCompartir
ShareCompartir