Progress Toward Measles Elimination — Nepal, 2007–2014
Weekly / March 4, 2016 / 65(8);206–210
Sudhir Khanal, MPH1; Tika Ram Sedai, MA1; Ganga Ram Choudary, MPH2; Jagat Narain Giri, MPH2; Rajendra Bohara, MD2; Rajendra Pant, MPH3; Mukunda Gautam, MPH3; Umid M. Sharapov, MD4; James L. Goodson, MPH4; James Alexander, MD4; Alya Dabbagh, PhD5; Peter Strebel, MBChB5; Robert T. Perry, MD5; Sunil Bah, MD1; Nihal Abeysinghe, MD1; Arun Thapa, MD1 (View author affiliations)
View suggested citationSummary
What is already known about this topic?
Before 2007, estimated coverage with the routine first dose of measles-containing vaccine (MCV1) in Nepal was ≤85% nationally; no districts had ≥95% MCV1 coverage, and measles was one of the major causes of childhood death.
What is added by this report?
During 2007–2014, MCV1 coverage increased from 71% to 88%; approximately 3.9 and 9.7 million children were vaccinated during supplemental immunization activities (SIAs) in 2008 and 2014, respectively; and annual suspected measles incidence declined 13%, from 54 to 47 cases per 1 million population. In 2013, a goal was set for measles elimination in Nepal by 2019. Challenges to achieving elimination include suboptimal MCV1 coverage at national and subnational levels and a low-performing measles case-based surveillance system.
What are the implications for public health practice?
Achieving ≥95% 2-dose measles vaccination coverage in all districts will require strengthening routine immunization services through innovative approaches, such as the “fully immunized village” approach, and implementing periodic high-quality SIAs. Improved measles case-based surveillance performance and sensitivity are needed for rapid case detection and outbreak preparedness and response.
In 2013, the 66th session of the Regional Committee of the World Health Organization (WHO) South-East Asia Region (SEAR) established a goal to eliminate measles and to control rubella and congenital rubella syndrome (CRS)* in SEAR by 2020 (1,2). Current recommended measles elimination strategies in the region include 1) achieving and maintaining ≥95% coverage with 2 doses of measles-containing vaccine (MCV) in every district, delivered through the routine immunization program or through supplementary immunization activities (SIAs)†; 2) developing and sustaining a sensitive and timely measles case-based surveillance system that meets minimum recommended performance indicators§; 3) developing and maintaining an accredited measles laboratory network; and 4) achieving timely identification, investigation, and response to measles outbreaks. In 2013, Nepal, one of the 11 SEAR member states, adopted a goal for national measles elimination by 2019 (3). This report updates a previous report (4) and summarizes progress toward measles elimination in Nepal during 2007–2014. During 2007–2014, estimated coverage with the first MCV dose (MCV1) increased from 81% to 88%. Approximately 3.9 and 9.7 million children were vaccinated in SIAs conducted in 2008 and 2014, respectively (1). Reported suspected measles incidence declined by 13% during 2007–2014, from 54 to 47 cases per 1 million population. However, in 2014, 81% of districts did not meet the measles case-based surveillance performance indicator target of ≥2 discarded non-measles cases¶ per 100,000 population per year. To achieve and maintain measles elimination, additional measures are needed to strengthen routine immunization services to increase coverage with MCV1 and a recently introduced second dose of MCV (MCV2**) to ≥95% in all districts, and to enhance sensitivity of measles case-based surveillance by adopting a more sensitive case definition, expanding case-based surveillance sites nationwide, and ensuring timely transport of specimens to the accredited national laboratory.
Immunization Activities
In 1979, monovalent measles vaccine was introduced as MCV1 in three districts in Nepal for vaccination of infants at age 9 months; in 1989, the program was scaled up nationally (5). After a nationwide SIA conducted in 2012–2013, measles-rubella (MR) vaccine was introduced into the national routine immunization schedule in 2013 and replaced monovalent measles vaccine as MCV1 administered at age 9 months. MCV2, in the form of MR vaccine, was introduced into the routine immunization program in September 2015 and is recommended for vaccination at age 15 months.
Administrative vaccination coverage data (number of vaccine doses administered divided by the estimated target population) are reported each year from the 75 districts in Nepal to the National Immunization Programme, where they are aggregated and reported to WHO and the United Nations Children’s Fund (UNICEF) through the Joint Reporting Form (JRF). WHO and UNICEF use reported administrative and immunization coverage survey data to estimate coverage through routine immunization services (6). In Nepal, estimated coverage with MCV1 increased from 81% in 2007 to 88% in 2014. In 2014, reported MCV1 coverage was <90% in 38 (51%) districts, 90%–94% in 15 (20%) districts, and ≥95% only in 22 (29%) districts.
To increase coverage, in 2011, Nepal initiated the “fully immunized village” concept, with the goal of achieving 100% coverage with all routinely recommended vaccines within the administrative boundary of each village using a strength-focused strategy called “appreciative inquiry,” a model that seeks to engage participants in self-determined change. In contrast to approaches for problem-solving that focus on deficiencies, appreciative inquiry offers processes and potential for the community to positively explore, collectively imagine, collaboratively design, and jointly commit to strengthening routine immunization and increasing MCV1 and MCV2 coverage. By 2014, a total of 823 (21%) of 3,915 villages and 10 (13%) districts were declared fully immunized, and a goal of having the entire country declared fully immunized through routine immunization services by 2017 was established (7).
During 2007–2014, two nationwide SIAs were conducted in phases. The first SIA, in 2008, used monovalent measles vaccine, reached 3.9 million children aged 9 months–4 years (971,470 and 2,932,045 during the first and second phases, respectively), and achieved 93% administrative coverage (Figure). The second nationwide SIA, conducted during 2012–2013, used MR vaccine, reached 9.7 million children aged 9 months–14 years (1,843,087; 2,203,863; and 5,638,149 during the first, second, and third phases, respectively), and achieved 100% administrative coverage.
Surveillance Activities and Measles Incidence
Suspected measles cases†† are reported from all health facilities through the national Health Management Information System (HMIS), and then compiled and reported to WHO and UNICEF through JRF (2). In 2003, measles case-based surveillance in Nepal was initiated as part of the Vaccine Preventable Disease (VPD) surveillance network on the existing acute flaccid paralysis surveillance system supported by WHO; data are provided from 735 reporting units, which include major health care centers and hospitals (approximately 10% of all government health facilities, including all inpatient facilities) throughout all 75 districts. During 2000–2006, case-based surveillance largely focused on investigation and reporting of suspected measles outbreaks. In 2007, enhanced measles case-based surveillance with individual case investigations and monitoring of performance indicators began; during 2007–2014, case-based surveillance expanded from 31 to 219 sites (4).
Cases are confirmed through the VPD surveillance network by outbreak investigations and case-based surveillance, with laboratory testing of specimens obtained from persons with suspected measles conducted by the National Public Health Laboratory in Kathmandu, the only WHO-accredited MR laboratory in the country. As a measure of surveillance sensitivity, the proportion of districts reporting ≥2 discarded non-measles cases per 100,000 population per year increased from 19% in 2007 to 52% in 2009. The increase could be attributed to an increase in detection and testing of non-measles cases through the VPD surveillance network that occurred during a large rubella outbreak that peaked in 2009. However, the proportion of districts reporting ≥2 discarded cases per 100,000 population declined to 19% again in 2014 (Table 1).
During 2007–2014, reported suspected measles incidence decreased 13%, from 54 to 47 cases per million population, based on aggregate data. During 2007–2014, a total of 10,047 suspected measles cases were reported through measles case-based surveillance, among which 2,849 (28%) were confirmed§§ (Table 2). The majority of confirmed cases occurred in children aged 9 months–4 years (44%) and 5–9 years (29%). Among confirmed measles cases, 45% had received ≥1 dose of MCV. After the 2012–2013 nationwide MR SIA, the number of reported confirmed measles cases declined by 98%, from 1,035 in 2011 to 25 in 2014. In 2014, among 347 suspected measles cases reported through case-based surveillance, 314 (84%) had serum specimens tested, and nine (3%) were laboratory confirmed as measles (Table 2). During 2007–2012, the reported measles virus genotypes in Nepal were D4 and D8; genotyping for cases detected during 2013–2014 was not done (8).¶¶
Discussion
During 2007–2014, reported incidence of suspected measles in Nepal decreased 13% after implementation of recommended elimination strategies. However, based on laboratory-confirmed and epidemiologically linked cases, the decline is likely much greater: only nine laboratory-confirmed cases were reported in 2014. The number of reported measles cases decreased after each nationwide SIA; these campaigns were carefully planned to prevent an accumulation of a large number of measles-susceptible persons, a situation that can result in large measles outbreaks (9).
Although reported routine MCV1 coverage in Nepal was 88% in 2014, 71% of districts reported <95% MCV1 coverage (approximately half reported <90% coverage). In addition to routine challenges to program improvement, natural disasters can also hinder measles elimination measures, with unanticipated interruptions of routine services and reprioritization of resources. For example, in April 2015, a massive earthquake caused major devastation and disrupted routine immunization services in affected districts. To sustain the gains achieved by the nationwide SIAs, the fully immunized village concept is being expanded to increase routine immunization coverage, and measles risk assessments are being conducted to identify and prioritize activities in low-performing districts (7).
A second dose of MCV was introduced into the routine immunization program nationwide in 2015 for vaccination of children at age 15 months. Achieving and sustaining measles elimination will require high levels of population immunity that can be achieved by reaching ≥95% coverage with both MCV1 and MCV2. Because the second dose is unique among currently recommended vaccines in Nepal, in that it is administered during the second year of life, it will take time, education, and outreach to achieve high attendance at this immunization visit and reach ≥95% coverage. Despite these challenges, the MCV2 visit will provide an opportunity to catch up on missed doses of vaccines recommended during the first year of life (especially MCV1) and a platform for introduction of future vaccines recommended during the second year of life.
High quality measles surveillance remains a challenge in Nepal. The WHO/UNICEF JRF data included totals of suspected cases (i.e. “clinically compatible” cases) from all health facilities (HMIS with aggregate case reporting), and most of those reported cases were not confirmed by laboratory testing or epidemiologic linkage to another confirmed case. Because measles case-based surveillance has only been implemented in 11% of health facilities (the VPD surveillance network), suspected cases are underreported through case-based surveillance. Key district-level surveillance indicators, such as the non-measles discarded case reporting rate, reflect underreporting and low sensitivity of the measles-specific case definition used in Nepal. The large number of clinically compatible cases reported in the case-based surveillance system indicates a failure to collect specimens for laboratory confirmation, in part because of challenges associated with transporting specimens to the laboratory in Kathmandu. Case-based surveillance sensitivity could be increased by expanding case-based surveillance to all health facilities in the country; by using a more sensitive rash-fever case definition; by potentially using alternative methods for specimen collection and transport, such as dried blood spots; and by increasing the collection and timely transport of specimens to the national laboratory. In addition, specimens for genotyping need to be collected on a proportion of measles and rubella cases to track transmission pathways, identify outbreak sources, and detect connections among cases.
The findings in this report are subject to at least two limitations. First, MCV1 coverage estimates can be affected by erroneous inclusion of SIA doses or doses administered to children outside the target group, inaccurate estimates of the target population size, and inaccurate reports of the number of doses delivered. Second, surveillance data might substantially underestimate disease incidence, because not all patients seek care, and not all patients who seek care are reported.
The endorsement in 2015 of the Nepal National Measles Elimination Strategy by the national government provides an opportunity to achieve and maintain measles elimination, by continuing to strengthen routine immunization services through innovative approaches, conducting high quality SIAs, enhancing case-based surveillance, increasing the number of specimens sent for laboratory confirmation, and identifying opportunities for synergies with other public health programs. In 2015, Nepal established the National Verification Committee for Measles Elimination, which is aligned with the global framework for the verification of progress toward measles elimination (10). As Nepal nears measles elimination, building capacity for epidemiologic investigations and outbreak preparedness and response to rapidly identify and contain outbreaks is needed. In addition to eliminating measles, these actions can enhance all aspects of the national public health system.
Corresponding author: Sudhir Khanal, khanals@who.int.
1Immunization and Vaccine Development (IVD), World Health Organization (WHO) South-East Asia Regional Office, Delhi, India; 2Immunization Preventable Disease, Nepal Country Office, WHO; 3Child Health Division, Department of Health Services, Ministry of Health and Population, Nepal; 4Global Immunization Division, Center for Global Health, CDC; 5IVD, WHO Headquarters, Geneva, Switzerland.
References
- World Health Organization Regional Office of South-East Asia. Strategic plan for measles elimination and rubella and congenital rubella syndrome control in the South-East Asia Region—2014–2020. New Delhi, India: World Health Organization, Regional Office for South East Asia; 2014. http://www.searo.who.int/entity/immunization/documents/sear_mr_strategic_plan_2014_2020.pdf
- Government of Nepal Department of Health Services Ministry of Health and Population. Measles elimination and rubella/congenital rubella syndrome control: national strategic plan 2015–19. Kathmandu, Nepal: Department of Health Services, Ministry of Health and Population, Government of Nepal; 2015.
- Suvedi BK. Immunisation programme of Nepal: an update. Kathmandu Univ Med J (KUMJ) 2004;2:238–43. PubMed
- CDC. Progress in measles control—Nepal, 2000–2006. MMWR Morb Mortal Wkly Rep 2007;56:1028–31. PubMed
- Suvedi BK. Twenty-five years of immunization program in Nepal. Kathmandu Univ Med J (KUMJ) 2005;3:4. PubMed
- World Health Organization; United Nations Children’s Fund. WHO/UNICEF estimates of national immunization coverage (WUENIC). Geneva Switzerland: World Health Organization; New York, NY: United Nations International Children’s Fund; 2015. http://www.who.int/immunization/monitoring_surveillance/data/en/
- World Health Organization Regional Office of South-East Asia. Appreciative inquiry: Nepal uses new approach to achieve full immunization for children. New Delhi, India: World Health Organization, Regional Office for South-East Asia; 2015. http://www.searo.who.int/mediacentre/events/appreciative-inquiry-story/en
- Rota PA, Brown K, Mankertz A, et al. Global distribution of measles genotypes and measles molecular epidemiology. J Infect Dis 2011;204(Suppl 1):S514–23. CrossRef PubMed
- World Health Organization. Measles vaccines: WHO position paper. Wkly Epidemiol Rec 2009;84:349–60. PubMed
- World Health Organization. Framework for verifying elimination of measles and rubella. Wkly Epidemiol Rec 2013;88:89–99. PubMed
* Measles elimination is defined as the absence of endemic measles cases for a period of ≥12 months, in the presence of adequate surveillance. One indicator of measles elimination is a sustained measles incidence of less than one case per million population. Rubella/CRS control is defined as 95% reduction in disease burden from 2013 levels.
† SIAs are immunization campaigns, typically carried out using two targeted age ranges. An initial, nationwide catch-up SIA targets all children aged 9 months–14 years, with the goal of eliminating measles susceptibility in the population. Periodic follow-up SIAs then target all children born since the last SIA. Follow-up SIAs generally are conducted every 2 to 4 years and target children aged 9–59 months; the goal of a follow-SIA is to eliminate any measles susceptibility that has accumulated in recent birth cohorts and to protect children who did not respond to the first dose of measles vaccine.
§ The SEAR measles surveillance indicators include 1) ≥2 discarded non-measles non-rubella cases per 100,000 population at the national level per year; 2) ≥2 discarded non-measles non-rubella cases per 100,000 per year in ≥80% of subnational administrative units; 3) ≥80% of suspected measles cases tested for measles immunoglobulin M antibodies; 4) ≥80% of suspected cases have an adequate investigation conducted within 48 hours of notification; 5) ≥80% of laboratory-confirmed chains of transmission have adequate samples collected for detecting measles or rubella virus and tested in an accredited laboratory; and 6) an annualized incidence rate of zero for confirmed endemic measles cases.
¶ A suspected case that has been investigated and identified as a non-measles case using testing in a proficient laboratory or epidemiologic linkage to a laboratory-confirmed outbreak of another communicable disease that is not measles.
** A second dose of MCV (MCV2) was introduced into the routine immunization program in September 2015 and is recommended at age 15 months.
†† A suspected measles case is defined as an illness in any person a clinician suspects of having measles infection, or in any person with fever and maculopapular rash, and cough, coryza, or conjunctivitis.
§§ Includes laboratory-confirmed, epidemiologically linked, and clinically compatible cases.
¶¶ In Nepal, specimens for genotyping were collected from cases once a measles outbreak was confirmed. Genotype data received from the Measles Nucleotide Surveillance database.
 FIGURE. Confirmed measles cases,* estimated coverage with the first dose of measles-containing vaccine (MCV1), and supplementary immunization activities (SIAs)†,§ — Nepal, 2007–2014
FIGURE. Confirmed measles cases,* estimated coverage with the first dose of measles-containing vaccine (MCV1), and supplementary immunization activities (SIAs)†,§ — Nepal, 2007–2014
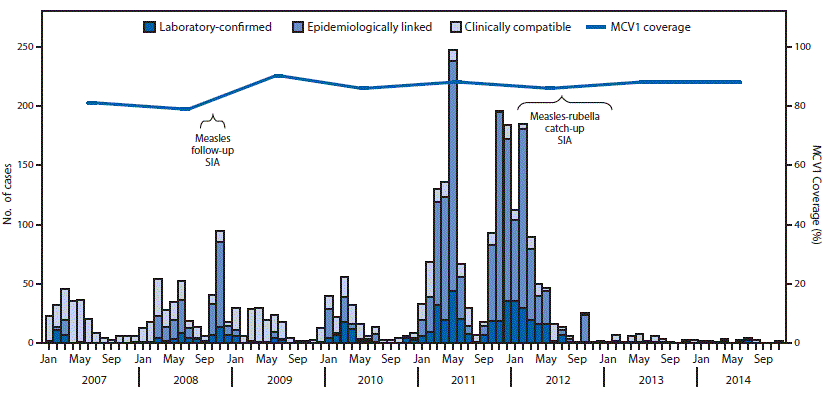
* Includes laboratory-confirmed, epidemiologically linked, and clinically confirmed, as reported through the national case-based surveillance system. National measles case-based surveillance system data as reported to WHO South-East Asia Region as of December 2015.
† The national measles follow-up SIA targeted children aged 9 months–4 years, implemented in two phases: the first phase was conducted from September 10, 2008, in 29 districts targeting 971,470 children; the second phase was conducted from December 6, 2008 in 46 districts targeting 2,932,045 children. The implementation period for each phase lasted a minimum of 9 days; the overall administrative coverage was 93%.
§ The national measles-rubella catch-up SIA targeted children aged 9 months–14 years, implemented in three phases: the first phase was conducted during February 26– March 25, 2012 in 15 districts in Far and Midwest Development Region (DR) targeting 1,843,087 children; the second phase was conducted during September 17–October 16, 2012 in 25 districts in Midwest and Western DR targeting 2,203,863 children; and the third phase was conducted during December 14, 2012–January 13, 2013 in 35 districts in Central and Eastern DR targeting 5,638,149 children. The overall reported administrative coverage was 100%.
 TABLE 1. National measles case-based surveillance performance indicator targets and progress — Nepal, 2007–2014
TABLE 1. National measles case-based surveillance performance indicator targets and progress — Nepal, 2007–2014
| Year | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Indicators | Target | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 |
| Reporting rate of discarded non-measles cases at the national level per year | ≥2 | 1.6 | 4.0 | 6.5 | 3.0 | 4.6 | 4.2 | 1.0 | 1.2 |
| Proportion of subnational administrative units (districts) reporting at least two discarded non-measles cases per 100 000 population per year | ≥80 | 19 | 35 | 52 | 37 | 49 | 45 | 16 | 19 |
| Percentage of suspected measles* cases adequately investigated† ≤48 hours of notification | ≥80 | 69 | 51 | 58 | 61 | 75 | 53 | 92 | 95 |
| Proportion of suspected cases with adequate specimens§ tested for measles in a proficient laboratory¶ | ≥80 | 48 | 44 | 52 | 60 | 41 | 46 | 85 | 84 |
| Proportion of results reported by the laboratory within 7 days of specimen receipt** | ≥80 | 69 | 94 | 88 | 84 | 84 | 62 | 88 | 97 |
| Proportion of weekly surveillance units reporting to the national level on time | ≥80 | 88 | 93 | 93 | 95 | 92 | 91 | 92 | 90 |
* A suspected measles case is defined as an illness in any person a clinician suspects of having measles infection, or in any person with fever and maculopapular rash and cough, coryza, or conjunctivitis.
† An adequate investigation includes collection of all the following data elements about each suspected case of measles or rubella: patient name or identifiers, place of residence, place of infection (at least to district level), age (or date of birth), sex, date of rash onset, date of specimen collection, measles-rubella vaccination status, date of last measles-rubella or measles-mumps-rubella vaccination, date of notification, date of investigation and travel history.
§ An adequate specimen is a blood specimen collected ≤28 days of the onset of rash.
¶ A proficient laboratory is one that is World Health Organization (WHO)-accredited and/or has an established quality assurance program with oversight by a WHO-accredited laboratory.
** Changed to 4 days in 2015.
 TABLE 2. Measles incidence,* number of reported measles cases by case classification, age group, and vaccination status — Nepal, 2007–2014
TABLE 2. Measles incidence,* number of reported measles cases by case classification, age group, and vaccination status — Nepal, 2007–2014
| Year | WHO/UNICEF JRF reporting† | Measles case-based surveillance§ | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case classification | Age group of confirmed measles cases No. (%) |
MCV doses received by confirmed measles case No. (%) | |||||||||||||
| No. reported suspected measles cases | Incidence (cases/million population) | No. suspected measles cases¶ | No. confirmed measles cases** (%) | No. laboratory-confirmed cases | No. epi linked cases | No. clinically compatible cases | <9 mos | 9 mos–4 yrs | 5–9 yrs | 10–14 yrs | ≥15 yrs | ≥1 | Zero | Unknown | |
| 2007 | 1,415 | 54 | 657 | 230 (35) | 22 | 16 | 192 | 23 (10) | 78 (34) | 64 (28) | 37 (16) | 28 (12) | 134 (58) | 47 (20) | 49 (21) |
| 2008 | 2,089 | 78 | 1,494 | 394 (26) | 61 | 188 | 145 | 32 (8) | 241 (61) | 80 (20) | 21 (5) | 20 (5) | 191 (48) | 165 (42) | 38 (10) |
| 2009 | 189 | 7 | 1,971 | 176 (9) | 20 | 11 | 145 | 7 (4) | 77 (44) | 59 (34) | 21 (12) | 12 (7) | 117 (66) | 31 (18) | 28 (16) |
| 2010 | 190 | 7 | 1,026 | 216 (21) | 53 | 62 | 101 | 28 (13) | 98 (45) | 53 (25) | 26 (12) | 11 (5) | 118 (55) | 76 (35) | 22 (10) |
| 2011 | 2,359 | 84 | 2,310 | 1,035 (45) | 190 | 719 | 126 | 45 (4) | 455 (44) | 290 (28) | 138 (13) | 107 (10) | 364 (35) | 505 (49) | 166 (16) |
| 2012 | 3,362 | 118 | 1,919 | 732 (38) | 166 | 497 | 69 | 21 (3) | 279 (38) | 257 (35) | 113 (15) | 62 (8) | 331 (45) | 321 (44) | 80 (11) |
| 2013 | 1,861 | 68 | 323 | 41 (13) | 10 | 0 | 31 | 10 (24) | 18 (44) | 8 (20) | 2 (5) | 3 (7) | 16 (39) | 22 (54) | 3 (7) |
| 2014 | 1,279 | 47 | 347 | 25 (7) | 9 | 0 | 16 | 5 (20) | 11 (44) | 5 (20) | 2 (8) | 2 (8) | 14 (56) | 8 (32) | 3 (12) |
Abbreviations: epi = epidemiologically; JRF = Joint Reporting Form; MCV = measles-containing vaccine; UNICEF = United Nations Children’s Fund; WHO = World Health Organization.
*Measles incidence calculated on the basis of reported suspected measles cases and population by member states through WHO/UNICEF JRF.
†National measles case data as reported to WHO South-East Asia Region Office (SEARO) as of December 2015 through the WHO/UNICEF JRF. Nepal uses administrative data reported through the national Health Management Information system (HMIS) to report in the JRF. The HMIS receives data from all the health facilities in the country, including private and public clinics and hospitals.
§ Data from case-based measles surveillance through the Vaccine Preventable Diseases surveillance network reported to WHO SEARO as of December 2015.
¶ A suspected measles case is defined as an illness in any person a clinician suspects of having measles infection, or in any person with fever and maculopapular rash and cough, coryza, or conjunctivitis.
** Includes laboratory-confirmed, epidemiologically linked, and clinically compatible cases. An epidemiologically linked case is one that meets the clinical case definition and is linked epidemiologically to a laboratory-confirmed or another epidemiologically confirmed case.
Suggested citation for this article: Khanal S, Sedai TR, Choudary GR, et al. Progress Toward Measles Elimination — Nepal, 2007–2014. MMWR Morb Mortal Wkly Rep 2016;65:206–210. DOI: http://dx.doi.org/10.15585/mmwr.mm6508a3.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (https://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.
- Page last reviewed: August 25, 2017
- Page last updated: August 25, 2017
- Content source:


 ShareCompartir
ShareCompartir