Notes from the Field: Kratom (Mitragyna speciosa) Exposures Reported to Poison Centers — United States, 2010–2015
Weekly / July 29, 2016 / 65(29);748–749
Mehruba Anwar, MD1; Royal Law, PhD1; Josh Schier, MD1 (View author affiliations)
View suggested citationKratom (Mitragyna speciosa) is a plant consumed throughout the world for its stimulant effects and as an opioid substitute (1). It is typically brewed into a tea, chewed, smoked, or ingested in capsules (2). It is also known as Thang, Kakuam, Thom, Ketum, and Biak (3). The Drug Enforcement Administration includes kratom on its Drugs of Concern list (substances that are not currently regulated by the Controlled Substances Act, but that pose risks to persons who abuse them), and the National Institute of Drug Abuse has identified kratom as an emerging drug of abuse (3,4). Published case reports have associated kratom exposure with psychosis, seizures, and deaths (5,6). Because deaths have been attributed to kratom in the United States (7), some jurisdictions have passed or are considering legislation to make kratom use a felony (8). CDC characterized kratom exposures that were reported to poison centers and uploaded to the National Poison Data System (NPDS) during January 2010–December 2015. The NPDS is a national database of information logged by the country’s regional poison centers serving all 50 United States, the District of Columbia, and Puerto Rico and is maintained by the American Association of Poison Control Centers. NPDS case records are the result of call reports made by the public and health care providers.
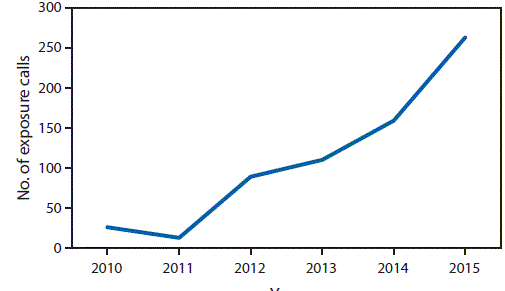
During the study period, U.S. poison centers received 660 calls about reported exposure to kratom. The number of calls increased tenfold from 26 in 2010 to 263 in 2015 (Figure). Health care provider reports constituted 496 (75.2%) of calls. Among calls, 487 (73.8%) exposed persons reported intentional exposure, and 595 (90.2%) reported ingestion of the drug. Isolated kratom exposure (single exposure) was reported in 428 (64.8%) cases. Among calls reporting use of kratom in combination with other substances (multiple exposures), the most commonly reported other substances were ethanol, other botanicals, benzodiazepines, narcotics, and acetaminophen. Among 658 (99.7%) calls for which information on sex of the exposed person was available, 472 (71.7%) were male, and among 604 (91.5%) for which information on age was available, the median age was 28 years (range = 2 months–69 years).
Medical outcomes associated with kratom exposure were reported as minor (minimal signs or symptoms, which resolved rapidly with no residual disability) for 162 (24.5%) exposures, moderate (non-life threatening, with no residual disability, but requiring some form of treatment) for 275 (41.7%) exposures, and major (life-threatening signs or symptoms, with some residual disability) for 49 (7.4%) exposures. One death was reported in a person who was exposed to the medications paroxetine (an antidepressant) and lamotrigine (an anticonvulsant and mood stabilizer) in addition to kratom. For 173 (26.2%) exposure calls, no effects were reported, or poison center staff members were unable to follow up again regarding effects. Among exposed persons for whom information on signs and symptoms was available, reported signs and symptoms included tachycardia (n = 165, 25.0%), agitation or irritability (157, 23.8%), drowsiness (128, 19.4%), nausea (97, 14.7%), and hypertension (77, 11.7%). A chi-square test demonstrated a significant association between severity of outcome and multiple versus single exposures (p<0.001). Pairwise comparisons (adjusted by the stepdown Bonferroni procedure) indicated a higher likelihood of a report of a severe outcome among persons aged 21–30 years (p = 0.04), 31–40 years (p = 0.02), and >40 years (p = 0.02) compared with persons aged 0–10 years.
Kratom use appears to be increasing in the United States (2), and the reported medical outcomes and health effects suggest an emerging public health threat. Members of the public and health care providers should be aware that the use of kratom can lead to severe adverse effects, especially when consumed in combination with alcohol or other drugs.
Corresponding author: Royal Law, rlaw@cdc.gov, 770-488-3416.
1Division of Environmental Hazards and Health Effects, National Center for Environmental Health, CDC.
References
- Neerman MF, Frost RE, Deking J. A drug fatality involving Kratom. J Forensic Sci 2013;58(Suppl 1):S278–9. CrossRef PubMed
- Warner ML, Kaufman NC, Grundmann O. The pharmacology and toxicology of kratom: from traditional herb to drug of abuse. Int J Legal Med 2016;130:127–38. CrossRef PubMed
- Drug Enforcement Administration. Drugs of abuse: a DEA resource guide. Springfield, VA: US Justice Department, Drug Enforcement Administration; 2015. http://www.dea.gov/pr/multimedia-library/publications/drug_of_abuse.pdf
- National Institute on Drug Abuse. DrugFacts: kratom. Bethesda, MD: National Institute on Drug Abuse; 2016. https://www.drugabuse.gov/publications/drugfacts/kratom
- Trakulsrichai S, Tongpo A, Sriapha C, et al. Kratom abuse in Ramathibodi Poison Center, Thailand: a five-year experience. J Psychoactive Drugs 2013;45:404–8. CrossRef PubMed
- Forrester MB. Kratom exposures reported to Texas poison centers. J Addict Dis 2013;32:396–400. CrossRef PubMed
- Coleman E. Anguished parents say exotic drug kratom is the cause of son’s suicide. Atlanta Journal Constitution. May 19, 2015. http://www.ajc.com/news/news/national/parents-warn-dangerous-substance-son-used-suicide/nmKmK/
- Pryor D. House panel votes to ban “kratom.” Sarasota Herald-Tribune. February 3, 2016. http://politics.heraldtribune.com/2016/02/03/house-panel-votes-to-ban-kratom
 FIGURE. Number of reported exposure calls to poison centers related to kratom use, by year — National Poison Data System, United States and Puerto Rico, January 2010–December 2015
FIGURE. Number of reported exposure calls to poison centers related to kratom use, by year — National Poison Data System, United States and Puerto Rico, January 2010–December 2015
Suggested citation for this article: Anwar M, Law R, Schier J. Notes from the Field. Kratom (Mitragyna speciosa) Exposures Reported to Poison Centers — United States, 2010–2015. MMWR Morb Mortal Wkly Rep 2016;65:748–749. DOI: http://dx.doi.org/10.15585/mmwr.mm6529a4.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (https://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.
- Page last reviewed: August 24, 2017
- Page last updated: August 24, 2017
- Content source:


 ShareCompartir
ShareCompartir