Status of New Vaccine Introduction — Worldwide, September 2016
Weekly / October 21, 2016 / 65(41);1136–1140
Anagha Loharikar, MD1; Laure Dumolard, PhD2; Susan Chu, PhD1; Terri Hyde, MD1; Tracey Goodman, MA2; Carsten Mantel, MD2 (View author affiliations)
View suggested citationSummary
What is already known about this topic?
Historically, new vaccines only became available in low-income and middle-income countries decades after being introduced in high-income countries. However, with support of global partners including the World Health Organization (WHO) and the United Nations Children’s Fund, which assist with vaccine prequalification and procurement, as well as Gavi, the Vaccine Alliance, which provides funding and shapes vaccine markets through forecasting and assurances of demand in low-income countries, in exchange for lower vaccine prices, vaccines are now introduced more rapidly.
What is added by this report?
As of September 2016, a total of 191 (99%) of 194 WHO member countries had introduced Hib vaccine, 190 (98%) had introduced hepatitis B vaccine, 132 (68%) had introduced pneumococcal conjugate vaccine (PCV), and 86 (44%) had introduced rotavirus vaccine into infant vaccination schedules. Human papillomavirus vaccine (HPV) had been introduced in 67 (35%) countries, targeted toward adolescent girls. A second dose of measles-containing vaccine (MCV2) had been introduced in 161 (83%) countries, and rubella vaccine had been introduced in 149 (77%).
What are the implications for public health practice?
Sustaining the health gains made through new vaccine introduction will require country commitment and funding, ensuring vaccine supply, creating and maintaining new age and target population delivery platforms, and addressing competing demands on health care systems and resources. New or improved vaccines for diseases such as meningitis, cholera, typhoid, malaria, and dengue will be available in the near future, bringing with them additional delivery and financing challenges along with the promise of decreased morbidity and mortality and the opportunity to further strengthen health systems.
Since the global Expanded Program on Immunization (EPI) was launched in 1974, vaccination against six diseases (tuberculosis, polio, diphtheria, tetanus, pertussis, and measles) has prevented millions of deaths and disabilities (1). Significant advances have been made in the development and introduction of vaccines, and licensed vaccines are now available to prevent 25 diseases (2,3). Historically, new vaccines only became available in low-income and middle-income countries decades after being introduced in high-income countries. However, with the support of global partners, including the World Health Organization (WHO) and the United Nations Children’s Fund, which assist with vaccine prequalification and procurement, as well as Gavi, the Vaccine Alliance (Gavi) (4), which provides funding and shapes vaccine markets through forecasting and assurances of demand in low-income countries in exchange for lower vaccine prices, vaccines are now introduced more rapidly. Based on data compiled in the WHO Immunization Vaccines and Biologicals Database* (5), this report describes the current status of introduction of Haemophilus influenzae type b (Hib), hepatitis B, pneumococcal conjugate, rotavirus, human papillomavirus, and rubella vaccines, and the second dose of measles vaccine. As of September 2016, a total of 191 (99%) of 194 WHO member countries had introduced Hib vaccine, 190 (98%) had introduced hepatitis B vaccine, 132 (68%) had introduced pneumococcal conjugate vaccine (PCV), and 86 (44%) had introduced rotavirus vaccine into infant vaccination schedules. Human papillomavirus vaccine (HPV) had been introduced in 67 (35%) countries, primarily targeted for routine use in adolescent girls. A second dose of measles-containing vaccine (MCV2) had been introduced in 161 (83%) countries, and rubella vaccine had been introduced in 149 (77%). These efforts support the commitment outlined in the Global Vaccine Action Plan (GVAP), 2011–2020 (2), endorsed by the World Health Assembly in 2012, to extend the full benefits of immunization to all persons.
Data on the status of vaccine introduction into the country’s routine immunization program, as of September 2016, were obtained from the WHO Immunization Vaccines and Biologicals Database, which receives vaccine introduction reports from 194 WHO countries. Vaccine introduction status is also presented by the 73 countries that were eligible† for support from Gavi for new vaccine introduction at any time since 2000 (5).
In 1992, WHO recommended hepatitis B vaccine as the first new vaccine in the childhood immunization schedule beyond the original EPI vaccines.§ Hepatitis B vaccine is now included in childhood immunization schedules in 190 (98%) countries, including 119 (61%) countries that have implemented a birth dose to prevent perinatal transmission of hepatitis B virus, as recommended by WHO in 2009 (6).
In 2000, Hib vaccine was only in widespread use in the WHO Region of the Americas and European Region. By 2006, when WHO recommended Hib vaccine in all routine infant immunization schedules, 108 countries, accounting for >55% of the world’s children, had introduced routine Hib vaccination (7). During the last decade, with continued WHO and Gavi support, expansion has continued and, as of September 2016, a total of 191 (98%) countries had incorporated Hib vaccine in national immunization schedules, including all 73 Gavi-eligible countries. Hib vaccine has not yet been introduced in China, the Russian Federation, and Thailand (5) (supplemental map 1, at https://stacks.cdc.gov/view/cdc/41681).
In 2007, WHO recommended use of PCV in all countries, prioritizing its introduction in countries with high pneumonia prevalence and high mortality rates among children aged <5 years. By 2008, although 24 high-income and two middle-income countries had initiated routine PCV vaccination, no countries in Africa or Asia, regions with high rates of pneumonia mortality among children aged <5 years, had yet introduced PCV (8). However, by September 2016, PCV had been introduced in national immunization schedules in 132 (68%) countries, and six more are planning introduction by the end of 2016. Among the 73 Gavi-eligible countries, 56 (77%) had introduced PCV, and three are planning introduction by the end of 2016 (5) (Figure 1).
Rotavirus vaccination has been recommended by WHO for inclusion in all national immunization schedules since 2009, particularly in countries with high rotavirus gastroenteritis–associated mortality, including South and Southeast Asia and sub-Saharan Africa. By September 2016, rotavirus vaccine had been introduced in 86 (44%) countries, including 38 (52%) Gavi-eligible countries. Five more countries plan to introduce rotavirus vaccine by the end of 2016 (5) (Figure 2).
Before 2012, HPV was not widely used outside North America, Australia, and Europe. In 2009, WHO recommended HPV for adolescent girls in all countries where cervical cancer prevention is a public health priority and introduction is feasible and sustainable. By September 2016, HPV had been introduced in 67 (35%) countries (5) (Figure 3). Among Gavi-eligible countries, 23 (32%) had conducted HPV pilot demonstration projects, and three had introduced HPV nationally.
Since 2009, WHO recommended 2 doses of MCV as the standard for all national immunization schedules (9). Routine MCV2 is usually administered during the second year of life, although in some countries it is scheduled around the age of school entry. As of September 2016, MCV2 had been introduced in 161 (83%) countries, and two countries have planned introduction in 2016 (5) (supplemental map 2, at https://stacks.cdc.gov/view/cdc/41682). Forty-six (63%) Gavi-eligible countries had introduced MCV2.
Rubella vaccine was first licensed in 1969 and initially was used primarily in high-income and middle-income countries. By 1996, only 85 countries included rubella vaccine in national immunization schedules. Since a 2011 Gavi commitment to support rubella vaccine introduction using combined measles-rubella vaccine, more countries have introduced rubella-containing vaccines, and, as of September 2016, rubella vaccine was included in national immunization schedules in 149 (77%) countries (5) (supplemental map 3, at https://stacks.cdc.gov/view/cdc/41683). Thirty-five (48%) Gavi-eligible countries had introduced rubella-containing vaccine in national immunization schedules.
Discussion
With the recognition of immunization as a core component of the human right to health, the availability of innovative funding mechanisms, and stronger international partnerships, vaccines are increasingly being introduced into national immunization schedules (2). Nearly all countries have introduced hepatitis B vaccine, more than three fourths have introduced rubella, MCV2 and Hib vaccines, and two thirds have introduced PCV. However, fewer than half have introduced rotavirus vaccine or HPV. In addition, market analyses indicate that recently introduced vaccines are reaching low-income countries faster than in the past. For example, Hib vaccine, introduced in 1989, took 20 years to reach 70% of low-income countries; PCV, introduced in 2000, is anticipated to reach 70% of low-income countries 5 years sooner (10). Although uneven, the overall success in extending vaccine introduction reflects both global commitment to achieving the GVAP goals and growing technical and resource capacities in low-income and middle-income countries.
Despite available resources from donors to introduce vaccines in countries, countries might choose not to introduce a particular vaccine because of national policies; financial constraints on implementation; lack of disease burden data; or vaccine hesitancy by the community, health system, or policy-makers. Establishing and strengthening independent advisory mechanisms at the national level (e.g., National Immunization Technical Advisory Groups) is critical for improving leadership in making informed and evidence-based recommendations about the introduction and financial sustainability of vaccines.
In countries where vaccines have been recently introduced into the national schedule, incomplete coverage might result in many children not receiving these vaccines. Immunization programs must closely review vaccine implementation and coverage to identify actions necessary to ensure equity and optimize impact. Continued progress in vaccine introduction will require national commitment and funding, ensuring vaccine supply and procurement, creating and maintaining new age and target population delivery platforms, and addressing competing demands on health care systems and resources. This is particularly critical for lower–middle-income countries and countries transitioning from eligibility for Gavi support, based on the World Bank data for gross national income.
Strategies that can help provide vaccine supply security include innovative pricing and procurement mechanisms, especially for lower–middle-income countries, as well as supply-side interventions and support for the manufacture of affordable vaccines in middle-income countries (1). Recent supply shortages of rotavirus, PCV, and other vaccines demonstrate the need to work with manufacturers at the global level to prevent stockouts (i.e., situations in which local vaccine providers run out of stock) or missed opportunities. Global and regional training initiatives targeting national programs have contributed to considerable improvements in vaccine stock management and cold-chain capacity; sustaining these infrastructure efforts will be important, particularly for vaccines that involve delivery and monitoring in new settings (e.g., school-based or outreach vaccination).
Vaccine introduction provides opportunities for strengthening a country’s immunization program and overall health system. Although vaccine introductions might require additional resources and innovative delivery strategies (e.g., school-based delivery of HPV, delivery of the hepatitis B birth dose as part of neonatal care), platforms for providing immunization to new age groups offer opportunities to improve access to health care and facilitate vaccination throughout the life course. Scheduled immunization visits provide a platform for integrating other public health interventions (e.g., vitamin A supplementation, deworming, bed nets for malaria prevention, and growth monitoring) to improve overall health. Delivering vaccination services beyond infancy (e.g., MCV2 in the second year of life) also offers an opportunity to provide vaccines missed during infancy to protect the child and improve vaccination coverage in the community. New partnerships established for vaccine introduction can enhance the delivery and efficiency of health services in new settings (e.g., ministries of education for school-based HPV vaccination) and strengthen traditional sites of service delivery. These partnerships also can support social and operational research and new communication strategies needed to increase acceptance of new vaccines by the target populations and among persons involved in the delivery of vaccines.
Sustaining the health gains made through vaccine introduction requires continued support for implementation, as well as support for surveillance to monitor disease burden, vaccine effectiveness, and vaccine safety. New or improved vaccines for diseases such as meningitis, cholera, typhoid, malaria, and dengue will be available in the near future, bringing with them additional delivery and financing challenges along with the promise of decreased morbidity and mortality and the opportunity to further strengthen health systems.
Acknowledgments
Kimberley Fox, MD, Abigail Shefer, MD, Global Immunization Division, Center for Global Health, CDC.
Corresponding author: Anagha Loharikar, igd2@cdc.gov, 404-718-6671.
1Global Immunization Division, Center for Global Health, CDC; 2Expanded Program on Immunization, World Health Organization.
* http://www.who.int/immunization/monitoring_surveillance/data/en.
† Gavi-eligible countries can choose to introduce a new vaccine without Gavi support. These data show Gavi eligibility, not necessarily Gavi support.
§ The original EPI vaccines protect against complications of tuberculosis in childhood (Bacille Calmette-Guérin [BCG] vaccine); diphtheria, tetanus, and pertussis (DTP vaccine); polio; and measles.
References
- Bland J, Clements J. Protecting the world’s children: the story of WHO’s immunization programme. World Health Forum 1998;19:162–73. PubMed
- World Health Organization. Global Vaccine Action Plan: 2011–2020. Geneva, Switzerland: WHO Press; 2013. http://www.who.int/immunization/global_vaccine_action_plan/en/
- World Health Organization. WHO recommendations for routine immunization—summary tables. Geneva, Switzerland: World Health Organization; 2015. http://www.who.int/immunization/policy/immunization_tables/en/
- Gavi, the Vaccine Alliance. Keeping children healthy: the Vaccine Alliance Progress Report 2015. Geneva, Switzerland: Gavi, the Vaccine Alliance; 2015. http://www.gavi.org/progress-report/
- World Health Organization. Immunization Vaccines and Biologicals Database, September 2016. Geneva, Switzerland: World Health Organization; 2016. http://www.who.int/immunization/monitoring_surveillance/data/en/
- World Health Organization. Hepatitis B vaccines. Wkly Epidemiol Rec 2009;84:405–19. PubMed
- Watt JP, Wolfson LJ, O’Brien KL, et al. ; Hib and Pneumococcal Global Burden of Disease Study Team. Burden of disease caused by Haemophilus influenzae type b in children younger than 5 years: global estimates. Lancet 2009;374:903–11. CrossRef PubMed
- O’Brien KL, Wolfson LJ, Watt JP, et al. ; Hib and Pneumococcal Global Burden of Disease Study Team. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 2009;374:893–902. CrossRef PubMed
- World Health Organization. A guide to introducing a second dose of measles vaccine into routine immunization schedules. Geneva, Switzerland: World Health Organization; 2013. http://www.who.int/immunization/documents/WHO_IVB_13.03/en/
- Vaccine Information Management System (VIMS) Report: Global Vaccine Introduction, December 2015. In: a report on current access to new childhood vaccines. Baltimore, MD: International Vaccine Access Center (IVAC), Johns Hopkins Bloomberg School of Public Health; 2015. http://www.jhsph.edu/research/centers-and-institutes/ivac/view-hub/IVAC-VIMS-Report-2015Dec.pdf
 FIGURE 1. Countries with current or planned use of pneumococcal conjugate vaccine (PCV) in the national immunization program, as of September 2016
FIGURE 1. Countries with current or planned use of pneumococcal conjugate vaccine (PCV) in the national immunization program, as of September 2016
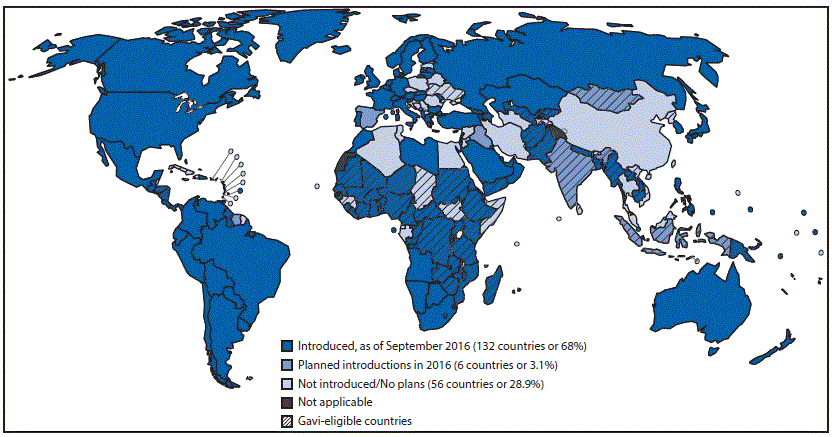
Source: World Health Organization, Immunization Vaccines and Biologicals Database, September 2016. http://www.who.int/immunization/monitoring_surveillance/data/en/.
Abbreviation: Gavi = Gavi, the Vaccine Alliance.
 FIGURE 2. Countries with current or planned use of rotavirus vaccine in the national immunization program, as of September 2016
FIGURE 2. Countries with current or planned use of rotavirus vaccine in the national immunization program, as of September 2016
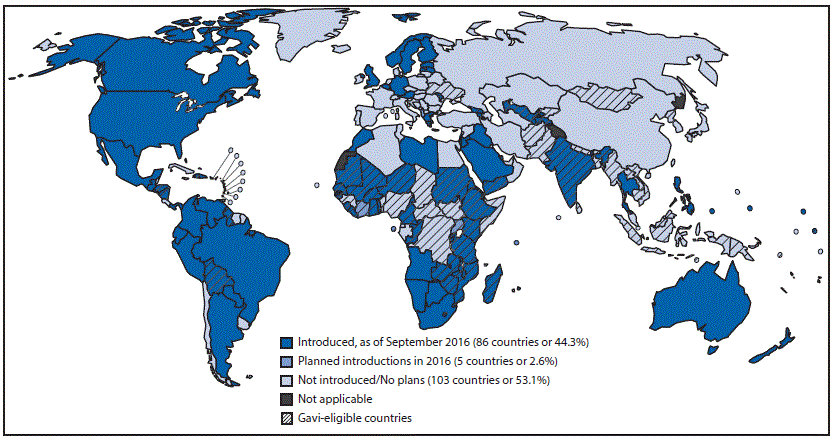
Source: World Health Organization, Immunization Vaccines and Biologicals Database, September 2016. http://www.who.int/immunization/monitoring_surveillance/data/en/.
Abbreviation: Gavi = Gavi, the Vaccine Alliance.
 FIGURE 3. Countries with current or planned use of human papillomavirus vaccine (HPV) in the national immunization program, as of September 2016
FIGURE 3. Countries with current or planned use of human papillomavirus vaccine (HPV) in the national immunization program, as of September 2016
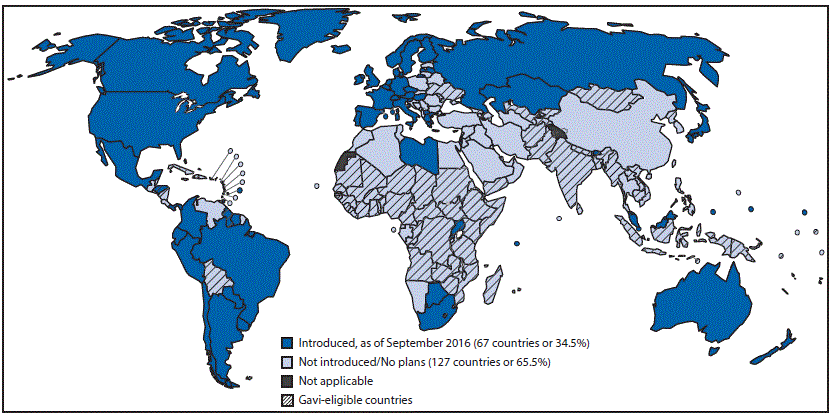
Source: World Health Organization, Immunization Vaccines and Biologicals Database, September 2016. http://www.who.int/immunization/monitoring_surveillance/data/en/.
Abbreviation: Gavi = Gavi, the Vaccine Alliance.
Suggested citation for this article: Loharikar A, Dumolard L, Chu S, Hyde T, Goodman T, Mantel C. Status of New Vaccine Introduction — Worldwide, September 2016. MMWR Morb Mortal Wkly Rep 2016;65:1136–1140. DOI: http://dx.doi.org/10.15585/mmwr.mm6541a3.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (https://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.
- Page last reviewed: August 17, 2017
- Page last updated: August 17, 2017
- Content source:


 ShareCompartir
ShareCompartir