Frequently Asked Questions

This page will help answer additional questions you may have about the Legacy program.
Is Legacy evidence-based?
Yes, the CDC conducted a multi-site, randomized controlled trial to examine Legacy’s impact on child health and development. Results revealed Legacy has a positive impact on child emotional and behavioral outcomes, child IQ, and mother-child interactions. The first outcome paper on Legacy published in the American Journal of Public Health (Kaminski et al., 2013) showed that children of mothers who did not participate in the Legacy program experienced an 80% higher likelihood of having clinically significant behavioral concerns.
In addition, Legacy is included in the National Registry for Evidence-based Programs and Practices and the Administration for Children and Families’ Compendium of Evidence-based Parenting Interventions.
Are there specific eligibility criteria to participate in Legacy ?
Currently CDC is working with the following federal agencies to implement Legacy: Administration for Children and Families’ Early Head Start, Health Resources and Services Administration’s Healthy Start, and Substance Abuse and Mental Health Administration’s Project LAUNCH. If a mother within one of these programs meets the socioeconomic risk requirement (e.g., Head Start eligible, Medicaid eligible), she is eligible to participate in a Legacy group. However, your organization should also clarify and screen for other factors that may affect program participation. For instance, who has custody of the child and/or who is the acting “parent?” Only one female family member with primary, physical custody can attend group sessions on behalf of a child.
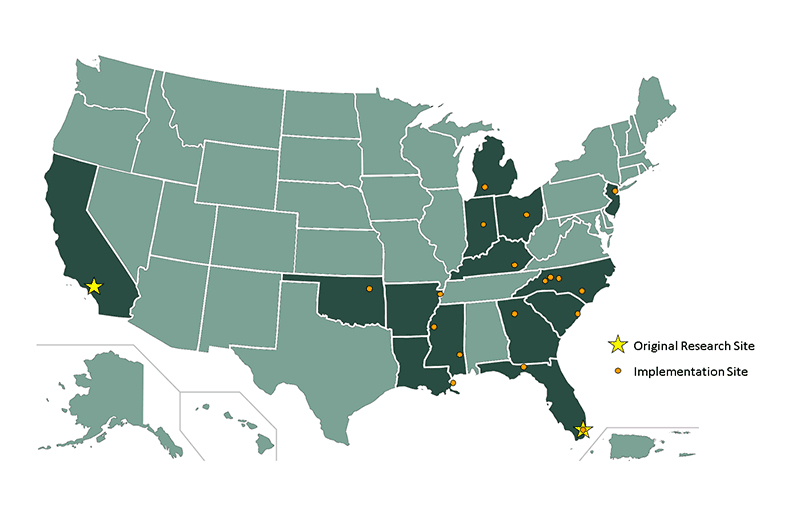
Where are other Legacy sites?
The first two Legacy sites started over 15 years ago at the University of California, Los Angeles (UCLA) and the University of Miami, Florida. CDC and partner organizations have now implemented within 14 states. View a map of past and current Legacy sites.
What age groups does Legacy serve?
The Legacy curriculum serves children from six weeks through five years of age (Miami curriculum) or prenatally through age three years (UCLA curriculum). Because both curricula are timed according to the developmental age of the child and contain themes repeated across years, groups should start at the beginning of the curriculum and limit the range of ages of the enrolled children to a few months whenever possible.
Why aren’t fathers included in Legacy groups?
At this time, CDC has not designed or tested Legacy for fathers. CDC designed and tested the Legacy model with mothers for several reasons. First, many of the primary caregivers in our target population are mothers. Second, the research to date indicates that mothers and fathers parent and build community in different ways. Third, CDC has learned from Legacy qualitative data that the Legacy groups are intimate places, and mixed-gender groups could significantly change the group dynamic. As it is currently written, the curriculum does not contain guidance on how to facilitate a mixed-gender group.
Because the Legacy curriculum was designed and tested for mothers, its evidence base only applies to mothers. We cannot determine if Legacy is the best possible intervention program for fathers. It is important to give both mothers and fathers the best possible programs; therefore, fathers should be referred to an evidence-based program designed for fathers. In the future, CDC would like to explore opportunities to pilot test a version of Legacy with fathers.
How do I choose a curriculum?
The Legacy program has two tested curriculum versions, one developed by the University of Miami and one developed by the University of California at Los Angeles. Both curricula were developed to adhere to the Legacy model. Both curricula were designed for mothers, reflect the Legacy goals, include developmentally appropriate parenting topics, are group-based with both mother-only and mother-child time, have one-on-one meetings between each mother and the Intervention Specialist, and have demonstrated, positive effects on child development outcomes. However, the curricula differ in terms of target age range (e.g., prenatal through three years versus birth through five years), number of sessions, session structure, and other implementation factors. The Legacy training process includes hands-on time for you to review both curriculum options, ask questions of CDC staff and technical assistance providers, and make a collaborative decision on which curricula will be the best fit for your agency, community, and staff.
Is it possible to make changes to the curriculum?
Some adaptations may be suitable; however, to ensure that they do not change the program in a way that could impact effectiveness, they should be discussed and verified as non-substantial changes by your Legacy technical assistance (TA) providers and CDC. For example, sites could exchange materials like videos or songs cited in the curriculum for similar materials. However, if there are changes to the Legacy message and evidence-based core model, it may have a negative impact on the effectiveness. CDC and your Legacy TA providers can help determine what is and is not consistent with the Legacy model. Legacy should be conducted as closely as possible to original design; for follow-up discussion, use your monitoring tools to collect ideas about what might be missing or what might have worked better for a specific group. Work with your Legacy TA provider if you are considering any changes to the curriculum, population, or format.
How do I know that my program is implemented with fidelity?
Legacy fidelity is monitored and supported in several ways: a) fidelity monitoring tools, b) individual TA and group calls, and c) site visits. The Legacy program includes fidelity monitoring tools that are completed after each group session. Fidelity monitoring tools are used to reflect on the session to make sure that the content was delivered as designed, and to allow for continuous, seamless quality improvement. Second, individual and group TA calls can be used to monitor and support fidelity implementation. Finally, all Legacy sites will have a site visit from their Legacy TA provider and/or CDC staff. Site visits help the Legacy TA providers and CDC gain a better picture of your community and what Legacy looks like at your organization. Site visits also help Legacy TA providers and CDC better understand the successes and challenges in Legacy implementation at your organization to better tailor and direct TA support.
Is Legacy available in other languages?
To increase accessibility of Legacy to a wider audience, curricula are currently being translated, adapted, and evaluated for Spanish speakers. Potential adaptations of Legacy for tribal communities are also being explored. Please return to this page for updates on Legacy adaptations and translations.
- Page last reviewed: July 5, 2017
- Page last updated: July 5, 2017
- Content source:
- Division of Human Development and Disabilities, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention



 ShareCompartir
ShareCompartir