Fatal Gastrointestinal Fungal Infection
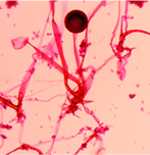
Photomicrograph of Rhizopus oryzae
In October 2014, CDC learned of an unusual case of fatal gastrointestinal mucormycosis in a premature infant. Gastrointestinal mucormycosis is a rare, serious infection that is thought to occur after a person ingests certain types of mold and usually only affects people with weakened immune systems.
An investigation by CDC, the Food and Drug Administration (FDA), and the Connecticut Departments of Public Health and Consumer Protection revealed that the infant had been given a dietary supplement called Solgar ABC Dophilus Powder. The supplement contained three bacteria which are believed to have probiotic effects. Laboratory testing at CDC on an unopened sample of the supplement also recovered the mold Rhizopus oryzae, the same mold that was found in a sample of the infant’s gastrointestinal tissue.
The supplement had been widely distributed in 29 states throughout the United States and to several other countries. CDC issued advice to consumers and healthcare providers to not use the supplement while the investigation was ongoing. The company voluntarily recalled the supplement, and no other cases of gastrointestinal mucormycosis were found.
More information
- Fatal Gastrointestinal Mucormycosis in an Infant Following Use of Contaminated ABC Dophilus Powder From Solgar Inc.
- Mucormycosis
- Notes from the Field: Fatal Gastrointestinal Mucormycosis in a Premature Infant Associated with a Contaminated Dietary Supplement — Connecticut, 2014. MWMR 2015; 64:155-156.
- Page last reviewed: August 7, 2015
- Page last updated: October 5, 2015
- Content source:


 ShareCompartir
ShareCompartir