We need you! Join our contributor community and become a WikEM editor through our open and transparent promotion process.
EBQ:ProCESS Trial
From WikEM
Complete Journal Club Article
The ProCESS Investigators. "A Randomized Trial of Protocol-Based Care for Early Septic Shock". NEJM. 2014. online first(online):11.
PubMed Full text PDF
PubMed Full text PDF
Contents
Clinical Question
In patients with severe sepsis in the Emergency Department dose protocol-based resuscitation improve outcomes
Conclusion
No mortality or morbidity benefit was found with protocol-based resuscitation compared to bedside care in patients with severe sepsis.
Major Points
- This multicenter randomized trial assigned patients 1341 patients to protocol EGDT (Early Goal Directed Therapy), protocol-based standard therapy that did not require the placement of a central venous catheter, administration of inotropes, or blood transfusions or usual care which was not standardized. The data was analyzed with intention to treat to determine in protocolized treatment of sepsis had a mortality benefit as was shown in the original Rivers Trial. No significant 60 or 90 day mortality benefit was found to the protocol therapy and greater hospital resources such as ICU admissions were found in the protocol based groups.
- There as an increased use of vasopressors, dobutamine, and blood transfusions in the protocol EGDT group. There was more volume given within 6 hours in the protocol-based standard therapy group. The EGDT group received more vasopressors and dobutamine, and blood transfusions than the other two groups.
- Ultimately sepsis care is improving with 2001 Rivers Trial morality of (30-40%) to this trial (18%).[1]
One important contribution of the ProCESS trial is the evidence it provides regarding the ongoing role of early recognition of and antibiotic treatment for sepsis in improving survival...The ProCESS trial identifies early recognition of sepsis, early administration of antibiotics, early adequate volume resuscitation, and clinical assessment of the adequacy of circulation as the elements we should focus on to save lives. The publication of the ProCESS trial launches the era of early recognition and treatment in the management of sepsis.—Craig M. Lilly, M.D, Editorial: The ProCESS Trial — A New Era of Sepsis Management[2]
Study Design
- Multicenter Randomized trial of 1241 patients (439 in protocol-based EGDT, 446 in protocol based standard therapy, and 456 in the usual-care group)
- The same physician led team implemented the EGDT and protocol based standard therapies
- The resuscitation guidelines were published in the supplemental document
- 31 Academic Hospitals
- Intention to treat statistical analysis
- Primary Outcome: All cause in hospital mortality at 60 days
Population
Inclusion Criteria
- Emergency Department Patients with suspected sepsis within 12 hour of ED arrival and 2 hours within detection of septic shock
- ≥18 years old
- Two SIRS Criteria
- Refractory Hypotension((SBP <90 mmHg or required vasopressors to maintain SBP≥90 mmHg after an IV fluid challenge) ) OR
- Serum Lactate ≥ 4 mmol/L
Participating Hospitals were required to have:
- >40,000 annual ED visits
- Use lactate as a screening measure for shock
- Not have a routine resuscitation protocol for septic shock
- Follow the Surviving Sepsis Campaign guidelines for non resuscitation aspects of care
- Not use ScvO2 catheters routinely
Exclusion Criteria
- Primary CVA
- Acute Coronary Syndrome
- Acute Pulmonary Edema
- Status Asthmaticus
- Major Cardiac Arrhythmia
- Acute GI Hemmorrhage
- Seizure
- Drug Overdose
- Burn or Trauma
- Immediate surgical requirement
- CD4 count < 50/mm2
- Advanced directive restricting protocol
- Central Venous Catheter contraindication
- Likelihood of refusing a blood transfusion
- Resuscitation deemed futile
- Participation in another interventional study
- Pregnancy
- Transfer to outside hospital
Patient Characteristics
Protocol Based EGDT group (n=439)
- Age: 60 years old
- Male: 52.8%
- From nursing home: 14.6%
- Top sepsis sources:
- Pneumonia 31.9%
- UTI 22.8%
- Intraabdominal 15.7%
- Unknown 13.0%
- Skin/soft tissue 5.7%
- Physiologic measurements:
- SBP: 100.2 mmHg
- Lactate: 4.8 mmol/L
- APACHE II: 20.8
- Positive blood culture: 31.7%
- Criteria for entry: Refractory hypotension 55.6%, elevated lactate 59.0%
- Time to randomization:
- From arrival in ED: 197 minutes
- From meeting inclusion criteria: 72 minutes
Protocol-Based Standard Therapy (n=446)
- Age: 61 years old
- Male: 56.5%
- From nursing home: 16.1%
- Top sepsis sources:
- Pneumonia 34.1%
- UTI 20.2%
- Intraabdominal 12.8%
- unknown 10.5%
- Skin/soft tissue 7.4%
- Physiologic measurements:
- SBP: 102.1 mmHg
- Lactate: 5 mmol/L
- APACHE II: 20.6
- Positive blood culture: 28.3%
- Criteria for entry: Refractory hypotension 53.8%, elevated lactate 59.2%
- Time to randomization:
- From arrival in ED: 185 minutes
- From meeting inclusion criteria: 66 minutes
Protocol-Based Standard Therapy (n=456)
- Age: 62 years old
- Male: 57.9%
- From nursing home: 16.%
- Top sepsis sources:
- Pneumonia 33.1%
- UTI 20.6%
- Intraabdominal 11.2%
- unknown 14.5%
- Skin/soft tissue 8.3%
- Physiologic measurements:
- SBP: 99.9 mmHg
- Lactate: 4.9 mmol/L
- APACHE II: 20.7
- Positive blood culture: 28.7%
- Criteria for entry: Refractory hypotension 53.3%, elevated lactate 60.7%
- Time to randomization:
- From arrival in ED: 181 minutes
- From meeting inclusion criteria: 69 minutes
Interventions
- Randomization to either:
Outcome
Intention to treat analysis of all cause mortality of Protocol EGDT, Protocol based Standard Therapy, and Physician Directed Usual Care
Primary Outcomes
- All-cause in-hospital mortality at 60 days
- EGDT: 21.0%
- Protocol based Standard Therapy: 18.2%
- Usual Care: 18.9%
- p=0.83
Secondary Outcomes
- All-cause mortality at 90 days
- EGDT: 31.9%
- Protocol based Standard Therapy: 30.8%
- Usual Care: 33.7%
- p=0.66
Subgroup analysis
- Use of Hospital Resources
- EGDT: 91.3%
- Protocol based Standard Therapy: 85.4%
- Usual Care: 86.2%
- p=0.01
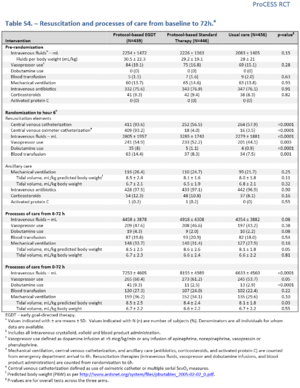
- Use of Intravenous Fluids (mL) randomization to hour 6hr
- EGDT: 2805±1957
- Protocol based Standard Therapy: 3285±1743
- Usual Care: 2279±1881
- p=0.0001
- Vasopressor Use randomization to hour 6hr
- EGDT:54.9%
- Protocol based Standard Therapy: 52.2%
- Usual Care: 44.1%
- p= 0.003
- Dobutamine Use randomization to hour 6hr
- EGDT: 8%
- Protocol based Standard Therapy: 1.1%
- Usual Care: 0.9%
- p=0.0001
- Blood Transfusions randomization to hour 6hr
- EGDT: 14.4%
- Protocol based Standard Therapy: 8.3%
- Usual Care: 7.5%
- p=0.001
- Corticosteroids randomization to hour 6hr
- EGDT: 12.3%
- Protocol based Standard Therapy: 10.8%
- Usual Care: 8.1%
- p=0.16
- Central Line randomization to hour 6hr
- EGDT: 93.6%
- Protocol based Standard Therapy: 56.5%
- Usual Care: 57.9%
- p= 0.0001
- Use of Intravenous Fluids (mL) 6-72 hr
- EGDT: 4458±3878
- Protocol based Standard Therapy: 4918±4308
- Usual Care: 4354 ± 3882
- p= 0.08
- Vasopressor Use 6-72hr
- EGDT: 54.9%
- Protocol based Standard Therapy: vs. 52.2%
- Usual Care: vs. 44.1%
- p= 0.003
- Dobutamine Use 6-72hr
- EGDT: 4.3%
- Protocol based Standard Therapy: 2.0%
- Usual Care: 2.2%
- p= 0.08
- Blood Transfusions 6-72hr
- EGDT: 19.8%
- Protocol based Standard Therapy: 20.9%
- Usual Care: 18.0%
- p=0.54
- Corticosteroids 6-72hr
- EGDT: 12.3%
- Protocol based Standard Therapy: 10.8%
- Usual Care: 8.1%
- p=0.16
Criticisms & Further Discussion
- Since sepsis education has advanced significantly since the 2001 Rivers Trial, the equalization of mortality may be due to the similar level of care given to patients regardless of protocol.
- A direct comparison cannot be made between the protocol EGDT group and the Rivers Trial because of greater severity of illness and persistence of shock and a small amount of non-compliance to the EGDT trial. Also usual care has advanced due to widespread sepsis education and the usual care in the Rivers Trial and the ProCESS trials are at different points of time in sepsis knowledge.
- The Surviving Sepsis Campaign initial response to the ProCESS Trial:[5]
- Importance of early recognition of sepsis with source control and early antibiotics
- 18% mortality rate in “usual care" is much better than the 46% seen in 2001 suggesting advances in sepsis care knowledge
- ProCESS does not answer the question about using a protocol to manage patients with severe sepsis without septic shock
- Supports MAP target of 65mmHg
- Repeat lactate testing not addressed in the ProCESS trial but other literate supports repeat testing
- Still encourages central lines as part of the 6hr bundle to check CVO2 since > 50% of the "usual care" patients received central lines
- The Surviving Sepsis Campaign response to Arise Trial and ProCESS Trial [6]
- Required monitoring of central venous pressure (CVP) and central venous oxygen saturation (ScvO2) via a central venous catheter (CVC) as part of an early resuscitation strategy does not confer survival benefit in all patients with septic shock who have received timely antibiotics and fluid resuscitation compared with controls.
- Requiring measurement of CVP and ScvO2 in all patients with lactate >4 mmol/L and/or persistent hypotension after initial fluid challenge who have received timely antibiotics is not supported by the available scientific evidence.
- The results of the ProCESS and ARISE trials have not demonstrated any adverse outcomes in the groups that utilized CVP and ScvO2 as end points for resuscitation. Therefore, no harm exists in keeping the current SSC bundles intact until a thorough appraisal of all available data has been performed.
Funding
- Scvo2 monitoring equipment for the study was loaned to the sites by Edwards Lifesciences, but the company had no other role in the study.
- University of Pittsburgh Clinical Research, Investigation, and Systems Modeling of Acute IllnessCenter managed all the data
- Funded by the National Institute of General Medical Sciences
- ClinicalTrials.gov: NCT00510835
See Also
Sources
- ↑ Rivers E, et al. "Early Goal Directed Therapy in the Treatment of Severe Sepsis and Septic Shock". The New England Journal of Medicine. 2001. 345(19):1368-1377
- ↑ www.nejm.org/doi/full/10.1056/NEJMe1402564
- ↑ Supplementary Appendix - EGDT flowsheet
- ↑ Supplementary Appendix - Standard Therapy flowsheet
- ↑ http://www.survivingsepsis.org/News/Pages/SSC-Responds-to-ProCESS-Trial.aspx PDF
- ↑ ../docss/SSCletterreversal.pdf