Hepatitis B FAQs for Health Professionals
Index of Questions
- What are the case definitions for reportable hepatitis B virus (HBV) infections?
- How many new HBV infections occur annually in the United States?
- Has the rate of new HBV infections in the United States declined?
- How common is chronic HBV infection in the United States?
- Where can I find more information about viral hepatitis incidence and prevalence in the United States?
± Transmission, Symptoms, and Treatment
- How is HBV transmitted?
- How long does HBV survive outside the body?
- What should be used to remove HBV from environmental surfaces?
- Who is at risk for HBV infection?
- Are international travelers at risk for HBV infection?
- What are the signs and symptoms of HBV infection?
- What is the incubation period for hepatitis B?
- When symptoms of acute hepatitis B occur, how long do they usually last?
- How serious is acute HBV infection?
- How serious is chronic HBV infection?
- How likely is HBV infection to become chronic?
- How is HBV infection treated?
- Who should be vaccinated against hepatitis B?
- Is hepatitis B vaccination recommended in certain settings?
- What are the hepatitis B vaccines licensed for use in the United States?
- What are the recommended schedules for hepatitis B vaccination?
- What are the recommended dosages of hepatitis B vaccines?
- Who should not receive hepatitis B vaccine?
- Can a patient receive the first dose of hepatitis B vaccine from one manufacturer and subsequent doses from another manufacturer?
- If there is an interruption between doses of hepatitis B vaccine, does the vaccine series need to be restarted?
- Is it harmful to administer an extra dose(s) of Hepatitis A or hepatitis B vaccine or to repeat the entire vaccine series if documentation of vaccination history is unavailable?
- Can hepatitis B vaccine be administered concurrently with other vaccines?
- How long does protection from hepatitis B vaccine last?
- Why should an infant receive hepatitis B vaccine at birth before hospital discharge, even if the mother is negative for hepatitis B surface antigen (HBsAg)?
- Can hepatitis B vaccine be given during pregnancy or lactation?
- Can hepatitis B vaccine be given to immunocompromised persons, such as persons on hemodialysis or persons with HIV infection?
- Can hepatitis B vaccine be given after exposure to HBV?
- Should persons be tested for immunity to hepatitis B before being vaccinated?
- Is there any benefit or risk in vaccinating a person who has been infected with HBV?
- Is postvaccination testing recommended? If so, for whom?
- When should postvaccination testing be done?
- Are booster doses of hepatitis B vaccine recommended?
Overview and Statistics
What are the case definitions for reportable hepatitis B virus (HBV) infections?
Case definitions have been developed by CDC, in collaboration with the Council of State and Territorial Epidemiologists, to provide uniform clinical and laboratory-testing criteria for the identification and reporting of nationally notifiable infectious diseases. The case definitions for acute, chronic, and perinatal hepatitis B are available at the following links:
Additional guidance on viral hepatitis surveillance and case management is available at https://www.cdc.gov/hepatitis/SurveillanceGuidelines.htm.
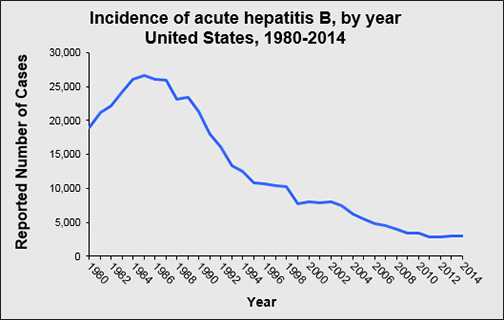
How many new HBV infections occur annually in the United States?
In 2014, a total of 2,953 cases of acute hepatitis B were reported from 48 states to CDC. The overall incidence rate for 2014 was 0.9 cases per 100,000 population. After adjusting for under-ascertainment and under-reporting, an estimated 19,200 acute hepatitis B cases occurred in 2014.
Has the rate of new HBV infections in the United States declined?
The rate of new HBV infections has declined by approximately 82% since 1991, when a national strategy to eliminate HBV infection was implemented in the United States. The decline has been greatest among children born since 1991, when routine vaccination of children was first recommended.
How common is chronic HBV infection in the United States?
An estimated 850,000–2.2 million persons in the United States have chronic hepatitis B virus infection. Chronic infection is an even greater problem globally, affecting approximately 240 million persons[1]. An estimated 786,000 persons worldwide die from HBV-related liver disease each year.[2]
Where can I find more information about viral hepatitis incidence and prevalence in the United States?
Viral hepatitis surveillance reports and guidelines are available at https://www.cdc.gov/hepatitis/statistics.htm.
Transmission, Symptoms, and Treatment
How is HBV transmitted?
HBV is transmitted through activities that involve percutaneous (i.e., puncture through the skin) or mucosal contact with infectious blood or body fluids (e.g., semen, saliva), including
- Sex with an infected partner
- Injection drug use that involves sharing needles, syringes, or drug-preparation equipment
- Birth to an infected mother
- Contact with blood or open sores of an infected person
- Needle sticks or sharp instrument exposures
- Sharing items such as razors or toothbrushes with an infected person
HBV is not spread through food or water, sharing eating utensils, breastfeeding, hugging, kissing, hand holding, coughing, or sneezing.
How long does HBV survive outside the body?
HBV can survive outside the body at least 7 days and still be capable of causing infection.
What should be used to remove HBV from environmental surfaces?
Any blood spills — including dried blood, which can still be infectious — should be cleaned using 1:10 dilution of one part household bleach to 10 parts of water for disinfecting the area. Gloves should be used when cleaning up any blood spills.
Who is at risk for HBV infection?
The following populations are at increased risk of becoming infected with HBV:
- Infants born to infected mothers
- Sex partners of infected persons
- Sexually active persons who are not in a long-term, mutually monogamous relationship (e.g., >1 sex partner during the previous 6 months)
- Men who have sex with men
- Injection drug users
- Household contacts of persons with chronic HBV infection
- Health care and public safety workers at risk for occupational exposure to blood or blood-contaminated body fluids
- Hemodialysis patients
- Residents and staff of facilities for developmentally disabled persons
- Travelers to countries with intermediate or high prevalence of HBV infection
Are international travelers at risk for HBV infection?
The risk for HBV infection in international travelers is generally low, except for certain travelers to regions where the prevalence of chronic HBV infection is high or intermediate (i.e., hepatitis B surface antigen prevalence of ≥2%). hepatitis B vaccination should be administered to unvaccinated persons traveling to those countries.
More information about hepatitis B and travel is available from CDC’s Travelers’ Health site.
What are the signs and symptoms of HBV infection?
The presence of signs and symptoms varies by age. Most children under age 5 years and newly infected immunosuppressed adults are asymptomatic, whereas 30%–50% of persons aged ≥5 years have initial signs and symptoms. When present, signs and symptoms can include
- Fever
- Fatigue
- Loss of appetite
- Nausea
- Vomiting
- Abdominal pain
- Dark urine
- Clay-colored bowel movements
- Joint pain
- Jaundice
Persons with chronic HBV infection might be asymptomatic, have no evidence of liver disease, or have a spectrum of disease ranging from chronic hepatitis to cirrhosis or hepatocellular carcinoma (a type of liver cancer).
What is the incubation period for hepatitis B?
Symptoms begin an average of 90 days (range: 60–150 days) after exposure to HBV.
When symptoms of acute hepatitis B occur, how long do they usually last?
Symptoms typically last for several weeks but can persist for up to 6 months.
How serious is acute HBV infection?
Acute infection ranges from asymptomatic or mild disease to — rarely — fulminant hepatitis. Disease is more severe among adults aged >60 years. The fatality rate among acute cases reported to CDC is 0.5%–1%.
How serious is chronic HBV infection?
Approximately 25% of those who become chronically infected during childhood and 15% of those who become chronically infected after childhood die prematurely from cirrhosis or liver cancer, and the majority remain asymptomatic until onset of cirrhosis or end-stage liver disease. In the United States, chronic HBV infection results in an estimated 1,800 deaths per year.
How likely is HBV infection to become chronic?
The risk for chronic infection varies according to the age at infection and is greatest among young children. Approximately 90% of infants and 25%–50% of children aged 1–5 years will remain chronically infected with HBV. By contrast, approximately 95% of adults recover completely from HBV infection and do not become chronically infected.
How is HBV infection treated?
For acute infection, no medication is available; treatment is supportive.
There are several antiviral medications for persons with chronic infection. Persons with chronic HBV infection require linkage to care with regular monitoring to prevent liver damage and/or hepatocellular carcinoma. AASLD Practice guidelines are available for the treatment of Chronic Hepatitis B and can be found on this site: http://www.aasld.org/publications/practice-guidelines-0.
Hepatitis B Serology
What do the different hepatitis B serologic markers mean?
hepatitis B surface antigen (HBsAg): A protein on the surface of HBV; it can be detected in high levels in serum during acute or chronic HBV infection. The presence of HBsAg indicates that the person is infectious. The body normally produces antibodies to HBsAg as part of the normal immune response to infection. HBsAg is the antigen used to make hepatitis B vaccine.
hepatitis B surface antibody (anti-HBs): The presence of anti-HBs is generally interpreted as indicating recovery and immunity from HBV infection. Anti-HBs also develops in a person who has been successfully vaccinated against hepatitis B.
Total hepatitis B core antibody (anti-HBc): Appears at the onset of symptoms in acute hepatitis B and persists for life. The presence of anti-HBc indicates previous or ongoing infection with HBV in an undefined time frame.
IgM antibody to hepatitis B core antigen (IgM anti-HBc): Positivity indicates recent infection with HBV (≤6 months). Its presence indicates acute infection.
hepatitis B e antigen (HBeAg): A secreted product of the nucleocapsid gene of HBV that is found in serum during acute and chronic hepatitis B. Its presence indicates that the virus is replicating and the infected person has high levels of HBV.
hepatitis B e antibody (HBeAb or anti-HBe): Produced by the immune system temporarily during acute HBV infection or consistently during or after a burst in viral replication. Spontaneous conversion from e antigen to e antibody (a change known as seroconversion) is a predictor of long-term clearance of HBV in patients undergoing antiviral therapy and indicates lower levels of HBV.
How do I interpret hepatitis B serologic test results?
The following table provides interpretations for hepatitis B serologic markers. A PDF version [PDF – 1 page] is also available.
|
Interpretation of hepatitis B Serologic Test Results
|
||
| Tests | Results | Interpretation |
|---|---|---|
| HBsAg anti-HBc anti-HBs |
negative negative negative |
Susceptible |
| HBsAg anti-HBc anti-HBs |
negative positive positive |
Immune due to natural infection |
| HBsAg anti-HBc anti-HBs |
negative negative positive |
Immune due to hepatitis B vaccination |
| HBsAg anti-HBc IgM anti-HBc anti-HBs |
positive positive positive negative |
Acutely infected |
| HBsAg anti-HBc IgM anti-HBc anti-HBs |
positive positive negative negative |
Chronically infected |
| HBsAg anti-HBc anti-HBs |
negative positive negative |
Interpretation unclear; four possibilities:
|
| hepatitis B surface antigen (HBsAg): A protein on the surface of HBV; it can be detected in high levels in serum during acute or chronic HBV infection. The presence of HBsAg indicates that the person is infectious. The body normally produces antibodies to HBsAg as part of the normal immune response to infection. HBsAg is the antigen used to make hepatitis B vaccine.
hepatitis B surface antibody (anti-HBs): The presence of anti-HBs is generally interpreted as indicating recovery and immunity from HBV infection. Anti-HBs also develops in a person who has been successfully vaccinated against hepatitis B. Adapted from: A Comprehensive Immunization Strategy to Eliminate Transmission of hepatitis B Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices. Part I: Immunization of Infants, Children, and Adolescents. MMWR 2005;54(No. RR-16). |
||
How long does it take for blood to test HBsAg-positive after exposure to HBV?
HBsAg will be detected in an infected person’s blood an average of 4 weeks (range: 1–9 weeks) after exposure to the virus. About 1 of 2 patients will no longer be infectious by 7 weeks after onset of symptoms, and all patients who do not remain chronically infected will be HBsAg-negative by 15 weeks after onset of symptoms.
Where can I learn more about viral hepatitis serology?
CDC offers an online training that covers the serology of hepatitis B and other types of viral hepatitis, available at https://www.cdc.gov/hepatitis/resources/professionals/training/serology/training.htm.
Hepatitis B Vaccination
Who should be vaccinated against hepatitis B?
The Advisory Committee on Immunization Practices recommends that the following persons be vaccinated against hepatitis B:
- All infants, beginning at birth
- All children aged <19 years who have not been vaccinated previously
- Susceptible sex partners of hepatitis B surface antigen (HBsAg)-positive persons
- Sexually active persons who are not in a long-term, mutually monogamous relationship (e.g., >1 sex partner during the previous 6 months)
- Persons seeking evaluation or treatment for a sexually transmitted disease
- Men who have sex with men
- Injection drug users
- Susceptible household contacts of HBsAg-positive persons
- Health care and public safety workers at risk for exposure to blood or blood-contaminated body fluids
- Persons with end-stage renal disease, including predialysis, hemodialysis, peritoneal dialysis, and home dialysis patients
- Residents and staff of facilities for developmentally disabled persons
- Travelers to regions with intermediate or high rates of endemic HBV infection
- Persons with chronic liver disease
- Persons with HIV infection
- Unvaccinated adults with diabetes mellitus who are aged 19 through 59 years (discretion of clinicians for unvaccinated adults with diabetes mellitus who are aged ≥60 years)
- All other persons seeking protection from HBV infection — acknowledgment of a specific risk factor is not a requirement for vaccination
Is hepatitis B vaccination recommended in certain settings?
Yes. In certain health care, evaluation, or treatment settings, a high proportion of clients have known risk factors for HBV infection. The Advisory Committee on Immunization Practices recommends universal vaccination of adults who receive care in those settings, including
- Sexually transmitted disease treatment facilities
- HIV testing and treatment facilities
- Facilities providing drug-abuse treatment and prevention services
- Health care settings targeting services to injection drug users
- Correctional facilities
- Health care settings targeting services to men who have sex with men
- Chronic hemodialysis facilities and end-stage renal disease programs
- Institutions and nonresidential day care facilities for developmentally disabled persons
What are the hepatitis B vaccines licensed for use in the United States?
Two single-antigen vaccines and three combination vaccines are currently licensed in the United States.
Single-antigen hepatitis B vaccines
- ENGERIX-B®
- RECOMBIVAX HB®
Combination vaccines
- PEDIARIX®: Combined hepatitis B, diphtheria, tetanus, acellular pertussis (DTaP), and inactivated poliovirus (IPV) vaccine. Cannot be administered before age 6 weeks or after age 7 years.
- TWINRIX®: Combined Hepatitis A and hepatitis B vaccine. Recommended for persons aged ≥18 years who are at increased risk for both Hepatitis A virus and HBV infections.
- COMVAX® (discontinued for purchase as of December 2014): Combined hepatitis B-Haemophilus influenzae type b (Hib) conjugate vaccine. Cannot be administered before age 6 weeks or after age 71 months.
What are the recommended schedules for hepatitis B vaccination?
The vaccination schedule most often used for children and adults is 3 intramuscular injections, the second and third doses administered 1 and 6 months, respectively, after the first dose. Alternate schedules have been approved for certain vaccines and/or populations.
What are the recommended doses of hepatitis B vaccines?
|
Recommended doses of currently licensed formulations of hepatitis B vaccine,
by age group and vaccine type |
|||||||||||
| Age Group | Single-antigen vaccine | Combination vaccine | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Recombivax HB | Engerix-B | Comvax* | Pediarix† | Twinrix§ | |||||||
| Dose (μg)¶ |
Vol(mL) | Dose (μg)¶ |
Vol(mL) | Dose (μg)¶ |
Vol (mL) | Dose (μg)¶ |
Vol (mL) | Dose (μg)¶ |
Vol (mL) | ||
|
Infants (<1 yr)
|
5 | 0.5 | 10 | 0.5 | 5 | 0.5 | 10 | 0.5 | NA** | NA | |
|
Children (1–10 yrs)
|
5 | 0.5 | 10 | 0.5 | 5* | 0.5 | 10† | 0.5 | NA | NA | |
|
Adolescents
|
11–15 yrs | 10†† | 1.0 | NA | NA | NA | NA | NA | NA | NA | NA |
| 11–19 yrs | 5 | 0.5 | 10 | 0.5 | NA | NA | NA | NA | NA | NA | |
|
Adults (≥20 yrs)
|
10 | 1.0 | 20 | 1.0 | NA | NA | NA | NA | 20§ | 1.0 | |
|
Hemodialysis patients
and other immuno- compromised persons |
<20 yrs§§ | 5 | 0.5 | 10 | 0.5 | NA | NA | NA | NA | NA | NA |
| ≥20 yrs | 40¶¶ | 1.0 | 40*** | 2.0 | NA | NA | NA | NA | NA | NA | |
* Combined hepatitis B–Haemophilus influenzae type b conjugate vaccine. This vaccine cannot be administered at birth, before age 6 weeks, or after age 71 months.
† Combined hepatitis B, diphtheria, tetanus, acellular pertussis adsorbed, inactivated poliovirus vaccine. This vaccine cannot be administered at birth, before age 6 weeks, or at age >7 years.
§ Combined Hepatitis A and hepatitis B vaccine. This vaccine is recommended for persons aged ≥18 years who are at increased risk for both hepatitis B virus and Hepatitis A virus infections.
¶ Recombinant hepatitis B surface antigen protein dose.
** Not applicable.
†† Adult formulation administered on a 2-dose schedule.
§§ Higher doses might be more immunogenic, but no specific recommendations have been made.
¶¶ Dialysis formulation administered on a 3-dose schedule at 0, 1, and 6 months.
*** Two 1.0-mL doses administered at one site, on a 4-dose schedule at 0, 1, 2, and 6 months.
Who should not receive hepatitis B vaccine?
Anyone who has had a serious allergic reaction to a prior dose of hepatitis B vaccine, a component of the hepatitis B vaccine, or yeast should not receive hepatitis B vaccine.
Can a patient receive the first dose of hepatitis B vaccine from one manufacturer and subsequent doses from another manufacturer?
Yes. No differences in immune response are observed when vaccines from different manufacturers are used to complete the vaccine series.
If there is an interruption between doses of hepatitis B vaccine, does the vaccine series need to be restarted?
No, the series does not need to be restarted.
- If the vaccine series was interrupted after the first dose, the second dose should be administered as soon as possible.
- The second and third doses should be separated by an interval of at least 8 weeks.
- If only the third dose is delayed, it should be administered as soon as possible.
Is it harmful to administer an extra dose(s) of Hepatitis A or hepatitis B vaccine or to repeat the entire vaccine series if documentation of vaccination history is unavailable?
No. If necessary, administering extra doses of Hepatitis A or hepatitis B vaccine is not harmful.
Can hepatitis B vaccine be administered concurrently with other vaccines?
Yes. When hepatitis B vaccine has been administered at the same time as other vaccines, no interference with the antibody response of the other vaccines has been demonstrated. Separate body sites and syringes should be used for simultaneous administration of injectable vaccines.
How long does protection from hepatitis B vaccine last?
Studies indicate that immunologic memory remains intact for at least 20 years among healthy vaccinated individuals who initiated hepatitis B vaccination >6 months of age. The vaccine confers long-term protection against clinical illness and chronic hepatitis B virus infection. Cellular immunity appears to persist even though antibody levels might become low or decline below detectable levels.
Among vaccinated cohorts who initiated hepatitis B vaccination at birth, long-term follow-up studies are ongoing to determine the duration of vaccine-induced immunity.
Why should an infant receive hepatitis B vaccine at birth before hospital discharge, even if the mother is negative for hepatitis B surface antigen (HBsAg)?
Infants born to HBV-infected mothers require hepatitis B vaccine and hepatitis B immune globulin (HBIG) within 12 hours of birth to protect them from infection. However, because errors or delays in documenting, testing, and reporting maternal HBsAg status can and do occur, administering the first dose of hepatitis B vaccine soon after birth to all infants acts as a safety net, reducing the risk for perinatal infection when maternal HBsAg status is either unknown or incorrectly documented at delivery. Also, initiating the hepatitis B vaccine series at birth has been shown to increase a child’s likelihood of completing the vaccine series on schedule.
Can hepatitis B vaccine be given during pregnancy or lactation?
Yes. hepatitis B vaccine contains no live virus, so neither pregnancy nor lactation should be considered a contraindication to vaccination of women. On the basis of limited experience, there is no apparent risk of adverse effects to developing fetuses when hepatitis B vaccine is administered to pregnant women. Meanwhile, new HBV infection in a pregnant woman might result in severe disease for the mother and chronic infection for the newborn.
Can hepatitis B vaccine be given to immunocompromised persons, such as persons on hemodialysis or persons with HIV infection?
Yes, although a larger vaccine dose is required to induce protective antibody in hemodialysis patients. Larger doses or additional doses might also be necessary for other immunocompromised persons. Serologic testing of hemodialysis patients and other immunocompromised persons is recommended 1–2 months after administration of the final dose of the primary vaccine series to determine the need for revaccination. Detailed guidance on vaccination of hemodialysis patients and other immunocompromised persons is available from the Advisory Committee on Immunization Practices recommendations on adult hepatitis B vaccination (available at https://www.cdc.gov/mmwr/PDF/rr/rr5516.pdf [PDF – 40 pages]).
Can hepatitis B vaccine be given after exposure to HBV?
Yes. After a person has been exposed to HBV, appropriate prophylaxis, given as soon as possible but preferably within 24 hours, can effectively prevent infection. The mainstay of postexposure immunoprophylaxis is hepatitis B vaccine, but in certain circumstances the addition of HBIG will provide increased protection.
Should persons be tested for immunity to hepatitis B before being vaccinated?
Routine prevaccination testing for immunity has not been recommended because it has not generally been found to be cost-effective. However, in adult populations that have an expected high prevalence (>20%) of HBV infection (e.g., IDUs and MSM, especially those in older age groups), conducting prevaccination serologic testing for susceptibility prior to or at the same time of the initial vaccine dose might be considered to be cost saving, by reducing the cost of the complete the vaccination series.
Prevaccination testing for susceptibility is recommended for foreign-born persons born in Africa, Asia, the Pacific Islands, and other regions with high endemicity of HBV infection (HBsAg prevalence of ≥8%); unvaccinated household, sexual, and needle-sharing contacts of HBsAg-positive persons; and for HIV-infected persons. Serologic testing should not be a barrier to vaccination. The first vaccine dose should be administered immediately after collection of the blood sample for serologic testing. Vaccination of persons who are immune to HBV infection because of current or previous infection or vaccination is not harmful and does not increase the risk for adverse events.
When a single test is used for prevaccination testing, anti-HBc is the test of choice. Persons who are anti-HBc–positive should be tested for HBsAg. If persons are determined to be HBsAg negative, no further action is required. Persons with positive HBsAg should be referred to a specialist in the management of hepatitis B infection and receive further serologic evaluation, prevention counseling, and evaluation for antiviral treatment (see Management of HBsAg-Positive Persons).
Is there any benefit or risk in vaccinating a person who has been infected with HBV?
Persons who have already been infected with HBV will receive no benefit from vaccination. However, there is no risk to a previously infected person who receives vaccination.
Who should receive postvaccination testing?
Testing for immunity is advised only for persons whose subsequent clinical management depends on knowledge of their immune status, including
- Infants born to HBsAg-positive mothers
- Health care workers and public safety workers at high risk for continued percutaneous or mucosal exposure to blood or body fluids
- Chronic hemodialysis patients, HIV-infected persons, and other immunocompromised persons (e.g., hematopoietic stem-cell transplant recipients or persons receiving chemotherapy)
- Sex partners of persons with chronic HBV infection
When should postvaccination testing be done?
For infants born to HBsAg-positive mothers, postvaccination testing should be performed 1–2 months after completion of ≥3 doses of a licensed hepatitis B vaccine series (i.e., at age 9–12 months, generally at the next well-child visit). To avoid detection of anti-HBs from hepatitis B immune globulin administered during infancy and to maximize detection of late HBV infection, testing should not be performed before age 9 months nor within 4 weeks of the most recent vaccine dose.
Are booster doses of hepatitis B vaccine recommended?
Booster doses of hepatitis B vaccine are recommended only in certain circumstances:
- For hemodialysis patients, the need for booster doses should be assessed by annual testing for antibody to hepatitis B surface antigen (anti-HBs). A booster dose should be administered when anti-HBs levels decline to <10 mIU/mL.
- For other immunocompromised persons (e.g., HIV-infected persons, hematopoietic stem-cell transplant recipients, and persons receiving chemotherapy), the need for booster doses has not been determined. When anti-HBs levels decline to <10 mIU/mL, annual anti-HBs testing and booster doses should be considered for those with an ongoing risk for exposure.
For persons with normal immune status who have been vaccinated, booster doses are not recommended.
References
- World Health Organization. Media Centre: hepatitis B. July 2013. Available at: http://www.who.int/mediacentre/factsheets/fs204/en/
- Lozano R, Naghavi M, Foreman K et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet 2012; 380: 2095-128.
- Page last reviewed: August 4, 2016
- Page last updated: August 4, 2016
- Content source:


 ShareCompartir
ShareCompartir