Early Identification and Prevention of the Spread of Ebola — United States
Supplements / July 8, 2016 / 65(3);75–84
Chris A. Van Beneden, MD1; Harald Pietz2; Robert D. Kirkcaldy, MD3; Lisa M. Koonin, DrPH4; Timothy M. Uyeki, MD5; Alexandra M. Oster, MD6; Deborah A. Levy, PhD7; Maleeka Glover, ScD8; Matthew J. Arduino, DrPH9; Toby L. Merlin, MD8; David T. Kuhar, MD9; Christine Kosmos, RN, MS2; Beth P. Bell, MD10 (View author affiliations)
View suggested citationSummary
In response to the 2014–2016 Ebola virus disease (Ebola) epidemic in West Africa, CDC prepared for the potential introduction of Ebola into the United States. The immediate goals were to rapidly identify and isolate any cases of Ebola, prevent transmission, and promote timely treatment of affected patients. CDC’s technical expertise and the collaboration of multiple partners in state, local, and municipal public health departments; health care facilities; emergency medical services; and U.S. government agencies were essential to the domestic preparedness and response to the Ebola epidemic and relied on longstanding partnerships. CDC established a comprehensive response that included two new strategies: 1) active monitoring of travelers arriving from countries affected by Ebola and other persons at risk for Ebola and 2) a tiered system of hospital facility preparedness that enabled prioritization of training. CDC rapidly deployed a diagnostic assay for Ebola virus (EBOV) to public health laboratories. Guidance was developed to assist in evaluation of patients possibly infected with EBOV, for appropriate infection control, to support emergency responders, and for handling of infectious waste. CDC rapid response teams were formed to provide assistance within 24 hours to a health care facility managing a patient with Ebola. As a result of the collaborations to rapidly identify, isolate, and manage Ebola patients and the extensive preparations to prevent spread of EBOV, the United States is now better prepared to address the next global infectious disease threat.
The activities summarized in this report would not have been possible without collaboration with many U.S. and international partners (http://www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/partners.html).
Background
As the epidemic of Ebola virus disease (Ebola) unfolded in West Africa in 2014, CDC prepared for the possible introduction of Ebola into the United States. The immediate objectives were to rapidly identify and isolate any cases of Ebola, prevent transmission of Ebola virus (EBOV), and ensure timely treatment of affected patients within the United States. CDC also sought to inform and prepare partners in the U.S. health care and public health systems.
In summer 2014, the lack of easy access to a diagnostic assay for EBOV complicated preparations for management of a patient with Ebola seeking care at any of the approximately 6,500 urgent-care clinics and 5,000 acute-care hospitals in the 50 states and the U.S. territories. Preparing the U.S. health care system to handle a rare but often fatal illness for which most clinicians and public health providers had no experience was daunting, particularly given the public’s expectation that there should be zero risk that a person who has Ebola could enter the country. Furthermore, providers needed to be educated on how to identify and isolate patients with suspected Ebola in a way that minimized the delay of appropriate medical care for more common and often serious illnesses (e.g., malaria) in travelers from West Africa.
Achieving readiness for the possibility that a person with Ebola could enter the United States required extensive collaboration with state and local public health officials, doctors and nurses in health care settings ranging from small clinics to large hospitals, hospital administrators, emergency responders, federal agencies, and transportation officials. This report describes the U.S. approach to achieving domestic Ebola readiness and response capacity and highlights key successes and unique challenges of the multiple facets of this process.
U.S. Preparations for Possible Importation of Ebola and the Impact of the First Confirmed Case
During summer 2014, while the Ebola epidemic raged approximately 5,000 miles away, CDC used health advisories and conference calls with public health partners and health care professionals to educate providers about Ebola and to encourage vigilance for imported cases of Ebola in the United States. On July 9, 2014, CDC activated its Emergency Operations Center (EOC), enabling a coordinated domestic and international response. Recognizing the need to diagnose Ebola quickly, CDC identified and distributed to state and local public health laboratories a laboratory assay that could reliably detect infection with the EBOV strain circulating in West Africa. CDC contacted the U.S. Department of Defense, which had an assay prepared for Emergency Use Authorization by the Food and Drug Administration, and worked with the Department of Defense and the Association of Public Health Laboratories to rapidly introduce and validate the assay in public health laboratories through the Laboratory Response Network (1).
In the early months of the EOC’s activation, CDC updated and posted prevention guidance developed for multiple audiences, including hospitals where travelers with suspected exposures to EBOV could seek care, emergency medical service providers, air medical transport operators, aircraft crew and airport personnel, laboratorians handling specimens from patients with suspected Ebola, and mortuary workers (Table 1). U.S. hospitals were considered to be capable of safely managing patients with Ebola (i.e., similar to the domestic experience treating patients with other viral hemorrhagic fevers, such as Marburg and Lassa) if recommendations for isolation of patients, appropriate use of personal protective equipment (PPE), and environmental cleaning and disinfection were followed.
On September 25, 2014, a man who had recently traveled to the United States from Liberia became symptomatic (i.e., fever, headache, and abdominal pain) and sought care at a hospital in Dallas, Texas. His illness was diagnosed as presumed sinusitis (2); he was treated and discharged to home (Table 2). On September 28, he was transported by ambulance to the hospital because of persistent fever and progressive symptoms and was hospitalized; on September 30, he became the first patient to have laboratory-confirmed EBOV infection diagnosed in the United States. Health officials from CDC and Texas subsequently identified 48 persons who had contact with him before his isolation at the hospital and began monitoring them for early signs of infection (3).
Within 7 days after the patient’s death, on October 8, Ebola symptoms developed in two nurses directly involved in his care, and they were confirmed to have Ebola (secondary cases) (2). Neither nurse reported an unprotected exposure to infectious blood or body fluids. A total of 147 health care workers who were involved in the care of the index patient or the two secondary cases (regardless of PPE used) were therefore closely monitored for 21 days after their last exposure to an Ebola patient (3). Ebola did not develop in any community or health care–related contacts of the three Ebola patients, including the family members with whom the index patient was living before hospitalization. Both nurses subsequently recovered (2).
Assisting the U.S. Clinical Community
After diagnosis of the three Ebola cases in Texas, requests for clinical consultation and general guidance from CDC increased, peaking at 227 calls per week in mid-October. The most frequent requests were for assistance in determining whether a patient fit the criteria for a person under investigation,* therefore warranting evaluation for Ebola. In most (75%) situations, the patients had no identifiable risk factors for Ebola (4). For these inquiries, CDC typically offered reassurance, confirming that the patient was actually not at risk for Ebola, and encouraged providers to provide timely routine medical care.
Patients who were isolated and evaluated for suspected Ebola were likely to experience delays in evaluation for and treatment of common but often serious (non-Ebola) illnesses. Basic diagnostic laboratory tests (e.g., complete blood counts, serum chemistries, malaria smears) and radiologic studies were often delayed for >2–3 days while patients were tested for EBOV (4). Although rapid identification and isolation (or transfer) of persons with suspected Ebola were important, so was the need to complete diagnostic testing quickly to enable proper management of other potentially life-threatening conditions (e.g., malaria, malignant hypertension, ectopic pregnancy) among persons arriving in the United States from West Africa (4).
Several reasons existed for this reluctance — or in some cases, refusal — to run basic diagnostic tests. The most recent (2009) U.S. Department of Health and Human Services (HHS) manual of biosafety (5) states that clinical specimens from persons with suspected Ebola should be manipulated only in a biosafety level (BSL)-4 facility, but most clinical laboratories are BSL-2. During the 2014–2016 Ebola epidemic, CDC updated its guidance for handling clinical specimens outside of a BSL-4 facility, but many laboratories considered the longstanding BSL-4 recommendation more appropriate. Also, clinical laboratories were concerned about the risk for aerosolization from instruments in highly automated clinical laboratories. Although CDC, and later other laboratories, provided guidance on conducting routine clinical laboratory tests using biosafety cabinets and point-of-care instruments (Table 1), many laboratories were not able to put these specialized systems in place.
CDC collaborated with other U.S. government partners, researchers, and manufacturers of medical countermeasures to assist health care providers with clinical management of Ebola patients in the United States. In early August 2014, Emory University Hospital (Atlanta, Georgia) hospitalized and treated the first Ebola patient medically evacuated to the United States (Table 2). During August 2014–March 2015, seven persons (six health care personnel and one journalist) who had Ebola diagnosed in West Africa were transported to the United States for clinical management; one died. These were in addition to two cases of Ebola diagnosed among persons traveling to the United States from countries affected by Ebola (the Dallas traveler and a health care worker who returned to New York City after working in Guinea) and the secondary EBOV infections in two nurses in Dallas (2,6). Extensive information sharing among clinicians managing these patients at the three specialized U.S. treatment centers,† Bellevue Hospital in New York City, Texas Health Presbyterian Hospital in Dallas, and hospitals in Europe contributed to substantial progress in understanding the clinical spectrum, complications, virology, and clinical management of Ebola, as well as the use of postexposure prophylaxis and medical countermeasures (2,7–11).
CDC’s outreach to clinicians included 1) directly assisting clinicians managing Ebola patients and Ebola survivors in the United States and sharing updated information with the general clinical community, including U.S. personnel deployed to the Monrovia Medical Unit in Liberia (12–15); 2) assisting with coordination of medical evacuations of Ebola patients who were U.S. citizens or legal permanent residents from West Africa to the specialized U.S. treatment centers (7–9); 3) working with clinical and federal partners to further the development of investigational therapeutic drugs for Ebola patients; and 4) coordinating information sharing among clinicians managing Ebola patients in the United States and Europe (16).
Ensuring Early Identification by Tracking Travelers and Tracking Contacts of Persons with Confirmed Ebola
During October 11–16, 2014, shortly after the patient from Liberia died, staff with CDC and the U.S. Department of Homeland Security’s Customs and Border Protection (CBP) began enhanced entry risk assessment and management at five U.S. airports that received approximately 94% of travelers from Guinea, Liberia, and Sierra Leone (17). This enhanced assessment followed growing concern that traveler self-monitoring might be insufficient to rapidly identify potential cases of Ebola (6). After travelers from countries affected by Ebola were screened for symptoms of Ebola and assigned an assessment of their personal risk, the responsibility for monitoring asymptomatic travelers for whom exposure to EBOV could not be ruled out and who were still in the 21-day incubation period was transferred from CDC to state and local public health partners. On October 21, 2014, CBP announced that all travelers from countries affected by Ebola were to be routed to one of five participating U.S. airports, enabling a standard approach to enhanced entry risk assessment of travelers and rendering the program more manageable.
CDC’s Interim U.S. Guidance for Monitoring and Movement of Persons with Potential Ebola Virus Exposure, initially issued in August 2014 and revised October 27,§ recommended that state, local, and territorial health agencies actively contact persons with specific risk factors for Ebola daily for the 21-day incubation period to assess them for symptoms and fever (18). Persons at low risk for Ebola (e.g., travelers from countries affected by Ebola without a known exposure) were asked to monitor their temperature twice a day, self-evaluate symptoms, and report daily to the designated health agency (active monitoring). Persons at high risk for exposure to EBOV (e.g., persons in contact with blood or other body fluids of known Ebola patients without proper PPE; health care workers who cared for patients even while using appropriate PPE) were to be under direct active monitoring; public health agencies conducted direct active monitoring for fever and symptoms twice daily, including direct observation by a public health official at least once a day. Each state and territory developed a plan to 1) monitor persons with possible EBOV exposure and locate those lost to follow-up and manage those who were noncompliant; 2) establish a 24/7 telephone number for persons with symptoms to call; 3) establish and practice systems (e.g., emergency medical services [EMS]) to ensure the safe transport of ill persons to a health care facility; and 4) identify the hospital to which a person would be referred should he or she become ill and ensure that the receiving health care facility was prepared at minimum to evaluate, isolate, and test (including collecting and shipping specimens) for Ebola.
Active monitoring of returning travelers and of health care providers and contacts of Ebola patients managed in the United States was a novel strategy introduced to facilitate early detection of new cases in the setting of no or minimal U.S. domestic transmission. Within 7 days after issuance of the revised CDC guidance in October 2014, all 50 states and two local jurisdictions were effectively monitoring travelers arriving from countries affected by Ebola and health care workers caring for Ebola patients in the United States (19). Approximately 29,000 persons were monitored from October 2014 through December 2015.
Nationwide implementation of this active monitoring system brought many challenges. Additional resources were needed to rapidly establish and staff 24/7 call numbers and to develop plans for effective daily observation of each person under direct active monitoring (including those living in remote places) (17). CDC awarded $145 million of supplemental Ebola funds to support the resulting substantial increase in staffing needs. Monitoring travelers moving across state lines required coordination among state health departments. Health departments and CDC were expected to achieve 100% accountability for all travelers; several health departments creatively used social media and police missing person units to find persons lost to follow-up. Also, a number of states elected to implement much more restrictive policies than recommended by CDC, resulting in inconsistencies among state-specific policies (6). Several states used existing laws requiring monitoring, with legal penalties for those not in compliance. For example, a nurse returning from treating patients in Sierra Leone (and asymptomatic) was quarantined in a New Jersey hospital for nearly 3 days (Table 2). Although the average rate of successful active monitoring reached approximately 99% by early March 2015 (19), this approach detected no new confirmed Ebola cases. Throughout this process, CDC maintained regular and frequent contact with partners to build a closer and better integrated response among federal, state, and local public health officials. During the height of the response, some federal public health partners embedded staff within CDC and the EOC.
On February 19, 2016, when more than 45 days had passed since Guinea was declared free of EBOV transmission and widespread human-to-human transmission was at an end, the interim guidance was retired. CDC will consider the need for similar guidance during future outbreaks on the basis of the situation, taking into account the extent of the outbreak and the risk of importation and spread of disease into the United States (18).
A Tiered Approach to Hospital Readiness
During the early phase of the epidemic in West Africa, any U.S. facility with trained staff, isolation room capacity, and appropriate supplies and equipment was considered capable of caring for a patient with Ebola. However, because of the complexity of care and strict attention to infection control (20) required for safe treatment of Ebola patients, highlighted by secondary EBOV transmission to the two nurses in Texas, CDC determined that ensuring adequately trained staff, availability of designated space, and adequate specialized PPE might not be possible in all inpatient facilities throughout the entire U.S. health care system. This level of preparation was critical for facilities most likely to receive patients for evaluation of Ebola. Also, the likelihood of a person with possible Ebola seeking care in an emergency department or hospital was not equally distributed among all hospitals in the United States for several reasons. Many travelers from West Africa lived in or visited specific regions of the country, travelers who were symptomatic on arrival to the United States were directed to specific hospitals near one of the five airports, and all persons under active monitoring by state public health officials could be directed to a particular hospital for evaluation if they developed symptoms during their monitoring period.
CDC and the HHS Office of the Assistant Secretary for Preparedness and Response (ASPR) developed a three-tiered approach to prepare U.S. acute health care facilities to safely and rapidly identify, isolate, evaluate, manage, and transfer (if needed) persons under investigation or patients with confirmed Ebola (21). The three tiers were frontline health care facilities, Ebola assessment hospitals, and Ebola treatment centers (Figure). CDC aimed to establish a limited number of Ebola treatment centers strategically in regions of the United States most likely to identify a person with Ebola.
Difficulties initially encountered included the few facilities with personnel trained to provide the complex care needed by Ebola patients, the limited number of facilities capable of managing children with Ebola, and a hesitancy of some facilities capable of providing care to Ebola patients to be identified publicly or to accept patients from other states. In addition, not all health care workers were trained in or familiar with using the specialized PPE recommended for care of Ebola patients. Some facilities struggled to identify dedicated space that was appropriately configured for Ebola management, and many facilities had substantial problems acquiring sufficient quantities and types of PPE (e.g., an Ebola treatment center should have a 5-day supply of PPE for a team of six nurses, three doctors, two laboratory technicians, two observers, and one environmental specialist for one to three shifts per day, depending on the health care worker’s role). Initially, PPE was ordered by facilities in high volumes with little strategic guidance, resulting in substantial delays in filling of orders and national shortages for some items. Manufacturers and distributors struggled to determine how much to increase production and how to prioritize orders and allocate limited resources.
CDC and ASPR, in collaboration with state and local public health authorities, produced detailed guidance for outpatient and inpatient facilities about managing persons under investigation and persons with confirmed Ebola (Table 1). Hospital Preparedness Program funding (22) was provided to states and eligible municipalities to improve surge capacity, including building needed infrastructure within health care systems, retrofitting hospitals to establish safe places to treat patients with Ebola, and reimbursement of care costs for confirmed Ebola patients. CDC also assembled Rapid Ebola Preparedness (REP) teams to assess infection control readiness of facilities interested in serving as Ebola treatment centers and provided on-site technical assistance regarding staffing, improvement in infection control, worker safety, laboratory processes, diagnostics, waste management, and other key areas. Initially, the REP teams provided direct technical assistance to hospitals near airports with a large number of persons traveling from countries that had widespread EBOV transmission and in communities where these travelers or large numbers of persons from West African countries reside. Beginning in October 2014, REP teams traveled to approximately 80 U.S. hospitals to provide technical support.
During October–December 2014, after extensive preparations, 55 hospital facilities were designated Ebola treatment centers by state health officers in collaboration with hospital administrators. These facilities received direct CDC and HHS technical assistance and formulated comprehensive plans outlining policies and procedures for managing patients with confirmed Ebola, which included training staff and instituting infection control measures, acquiring equipment and PPE, creating plans for managing waste, and designating appropriate space to treat Ebola patients. By August 2015, 92% of persons being monitored were within 200 miles of an Ebola treatment center and within 50 miles of an assessment hospital.
CDC’s Ebola Response Teams
To improve the response capacity to EBOV infections in the United States, CDC established teams capable of rapidly providing on-site assistance to any health care facility treating a confirmed or probable case of Ebola. These CDC Ebola response teams could be immediately deployed to provide technical assistance for infection control procedures, clinical care, logistics of managing a patient with Ebola, contact tracing, and media relations (23).
Emergency Medical Services
Success of the three-tiered health care system plan rested on safe and rapid transport of a person under investigation or patient with confirmed Ebola to a designated facility to be evaluated or treated. EMS responders faced multiple challenges, such as the potential to enter uncontrolled environments including homes and public areas with little or no information about the patient’s risk factors and the need to transport patients over long distances during which the patient’s condition could worsen. Lack of experience with Ebola and limited access to appropriate PPE encountered early in the U.S. response compounded these challenges.
CDC collaborated with federal partners to rapidly develop guidance for EMS systems and 9-1-1 public safety answering points for managing persons under investigation or patients with confirmed Ebola (Table 1). CDC also hosted conference calls to provide a forum for EMS providers from Emory University Hospital and the University of Nebraska Medical Center to share their experiences transporting Ebola patients. Further guidance addressing the complexities of interfacility and interstate transport of persons under investigation and patients with confirmed Ebola was developed in collaboration with ASPR and the U.S. Department of Transportation (DOT), National Highway Traffic Safety Administration (Table 1).
Environmental and Waste Management
All levels of health care facilities and EMS providers needed plans for the transport and disposal of waste generated by either persons under investigation or persons with confirmed Ebola. Fear, public perception, and the regulatory framework around handling Ebola-associated wastes proved to be common issues. These issues were encountered in health care facilities, patients’ homes, businesses that the patients frequented early in their disease, and a commercial passenger aircraft on which one patient flew while ill.
Although EBOV is susceptible to both physical and chemical inactivation, it is classified as a category A infectious substance¶ because of its associated high mortality rate. Therefore, items that are or might be contaminated must be treated onsite or packaged and transported to a hazardous waste or medical waste treatment site by a carrier with a special DOT permit. Once treated, the waste is no longer infectious and can be managed in accordance with state and local regulations regarding solid wastes. Unforeseen was the volume of waste generated, most of which was used PPE, and the packaging required for the waste because the packaging used was too large for the doors of most incinerators.
During the Ebola response, CDC collaborated with federal and state agencies and multiple other private and nongovernmental organizations to develop guidance for cleaning and disinfection applicable to various settings that included patient residences, commercial passenger and medical transport aircraft, ambulances, and health care facilities. Other guidance covered handling of medical, laboratory, liquid, and other wastes and the protection of waste handlers and sewage and wastewater workers from contact with untreated human wastes (Table 1).
Conclusion
Coordination of preparedness efforts among CDC and state and local public health entities, health care organizations, and other HHS partners, the product of longstanding partnerships, was central to the rapid implementation of a comprehensive U.S. domestic response. The United States quickly deployed laboratory testing for EBOV. The closely integrated system of U.S. border entry risk assessment and postarrival monitoring was pivotal to reducing public concern and facilitating active, timely management of symptomatic travelers. Vulnerabilities in infection control capacity exposed during the early outbreak response resulted in ongoing intensive efforts for improvements at the national, state, and local levels. The importance of support functions (e.g., waste management, laboratory testing, and EMS), which are needed to successfully care for patients with a complex, unfamiliar, and often fatal disease such as Ebola, have been underscored. The tiered approach to health care preparedness for Ebola highlighted the critical functions needed at each level and made possible the prioritization of training and other interventions. This tiered approach is likely to be transferable to the next public health response to future threats; nine regional treatment centers designated by HHS to become special regional treatment centers for patients with Ebola have enhanced capabilities that can be used to treat patients with other severe, highly infectious diseases. The United States is now better prepared and continues to work to strengthen and support rapid and successful responses to the next infectious disease threat.
Acknowledgments
Steve Boedigheimer, Mark Davis, Todd Talbert, and others on the State Coordination Task Force and the Monitoring and Movement Unit; John Brooks, Denise Jamieson, Cynthia Whitney, Dana Meaney-Delman, Peter Briss, Fleetwood Loustalot, Sue Gerber, John Jernigan, Joe Perz, Jeff Hagemann, and others on the Medical Care Task Force and Infection Control Teams; Barbara Mahon, Paul Mead, Barbara Knust, Stephanie Bialek, Alicia Fry, Monina Klevens, Margaret (Peggy) Honein, Lyn Finelli, Gayle Langley, Brett Peterson, Diana Blau, and others on the Domestic Epidemiology Team; Elissa Meites, Emily Koumans, Roxanne Barrow, Mary Kamb, LeAnne Fox, and others assisting with domestic clinical inquiries; Stephanie Schrag, Alex Kallen, Chris Braden, Todd Weber, Lyle Petersen, Matt Moore, Carrie Reed, and the many persons who worked on the ground in Texas, Ohio, and New York City; Jean Randolph, Amy Valderrama, Kelly Dickinson, Sherline Lee, and others on the Healthcare Systems Response Team; members of the Clinical Team; Lisa Delaney, Renee Funk, Chad Dowell, Jill Shugart, and others on the Worker Safety and Health Team and with CDC’s National Institute for Occupational Safety and Health; Anita Patel, Domestic PPE Lead; Nicki Peski, Nicole Cohen, Clive Brown, and others on the Global Migration Task Force; Ute Stroeher, Mark Rayfield, and the Laboratory Team; Chiefs of Staff Sherrie Bruce, David Hutchins, Peter Rzeszotarski, Toby Crafton, Elizabeth Bell, Serena Fuller, John Turner, and Stephen Papagiotas in particular for his contributions to the CDC Ebola Response Team; Jeff Nemhauser and Lauri Hicks for leading efforts to assist CDC staff returning from West Africa; Ed Rouse, Reed Sheridan, and the EOC Logistics Team; Inger Damon, Jordan Tappero, Eric Kasowski, and other incident managers and deputy incident managers; Julie Guarnizo, Beth Schweitzer, Rachel Holloway, Paula Rosenberg; and CDC Ebola response staff in Atlanta and in affected countries.
Corresponding author: Chris A. Van Beneden, Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, CDC. Telephone: 404-639-3575; E-mail: CVanBeneden@cdc.gov.
1Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, CDC; 2Division of State and Local Readiness, Office of Public Health Preparedness and Response, CDC; 3Division of STD Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, CDC; 4Office of the Director, National Center for Immunization and Respiratory Diseases, CDC; 5Influenza Division, National Center for Immunization and Respiratory Diseases, CDC; 6Division of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, CDC; 7Division of State and Local Readiness, Office of Public Health Preparedness and Response, CDC; 8Division of Preparedness and Emerging Infections, National Center for Emerging and Zoonotic Infectious Diseases, CDC; 9Division of Healthcare Quality Promotion, National Center for Emerging and Zoonotic Infectious Diseases, CDC; 10National Center for Emerging and Zoonotic Infectious Diseases, CDC
References
- CDC. The Laboratory Response Network partners in preparedness. http://emergency.cdc.gov/lrn/index.asp
- Liddell AM, Davey RT , Mehta AK, et al. Characteristics and clinical management of a cluster of 3 patients with Ebola virus disease, including the first domestically acquired cases in the United States. Ann Intern Med 2015;163:81–90. CrossRef PubMed
- Chevalier MS, Chung W, Smith J, et al. Ebola virus disease cluster in the United States—Dallas County, Texas, 2014. MMWR Morb Mortal Wkly Rep 2014;63:1087–8. PubMed
- Karwowski MP, Meites E, Fullerton KE, et al. Clinical inquiries regarding Ebola virus disease received by CDC—United States, July 9–November 15, 2014. MMWR Morb Mortal Wkly Rep 2014;63:1175–9. PubMed
- US Department of Health and Human Services. Biosafety in microbiological and biomedical laboratories. 5th edition. Atlanta, GA: CDC; 2009. HHS publication no. (CDC) 21–1112:238.
- Spencer C. Having and fighting Ebola—public health lessons from a clinician turned patient. N Engl J Med 2015;372:1089–91. CrossRef PubMed
- Lyon GM, Mehta AK, Varkey JB, et al. Clinical care of two patients with Ebola virus disease in the United States. N Engl J Med 2014;371:2402–9. CrossRef PubMed
- Kraft CS, Hewlett AL, Koepsell S, et al. The use of TKM-100802 and convalescent plasma in 2 patients with Ebola virus disease in the United States. Clin Infect Dis 2015;61:496–502. CrossRef PubMed
- Florescu DF, Kalil AC, Hewlett AL, et al. Administration of brincidofovir and convalescent plasma in a patient with Ebola virus disease. Clin Infect Dis 2015;61:969–73. CrossRef PubMed
- Lai L, Davey R, Beck A, et al. Emergency postexposure vaccination with vesicular stomatitis virus-vectored Ebola vaccine after needlestick. JAMA 2015;313:1249–55. CrossRef PubMed
- Wong KK, Davey RT , Hewlett AL, et al. Use of post-exposure prophylaxis after occupational exposure to Zaire ebolavirus. Clin Infect Dis 2016;ciw256. CrossRef PubMed
- Wong KK, Perdue CL, Malia J, et al. Supportive care of the first 2 Ebola virus disease patients at the Monrovia Medical Unit. Clin Infect Dis 2015;61:e47–51. CrossRef PubMed
- Varkey JB, Shantha JG, Crozier I, et al. Persistence of Ebola virus in ocular fluid during convalescence. N Engl J Med 2015;372:2423–7. CrossRef PubMed
- Epstein L, Wong KK, Kallen AJ, Uyeki TM. Post-Ebola signs and symptoms in U.S. survivors. N Engl J Med 2015;373:2484–6. CrossRef PubMed
- Uyeki TM, Erickson BR, Brown S, et al. Ebola virus persistence in semen of male survivors. Clin Infect Dis 2016;ciw202. CrossRef PubMed
- Uyeki TM, Mehta AK, Davey RT , et al. ; Working Group of the U.S.–European Clinical Network on Clinical Management of Ebola Virus Disease Patients in the U.S. and Europe. Clinical management of Ebola virus disease in the United States and Europe. N Engl J Med 2016;374:636–46. CrossRef PubMed
- Cohen NJ, Brown CM, Alvarado-Ramy F, et al. Travel and border health measures to prevent the international spread of Ebola. In: CDC response to the 2014–2016 Ebola epidemic—West Africa and United States. MMWR Suppl 2016;65(No. Suppl 3).
- CDC. Interim U.S. guidance for monitoring and movement of persons with potential Ebola virus exposure. Atlanta, GA: US Department of Health and Human Services, CDC; 2015. http://www.cdc.gov/vhf/Ebola/exposure/monitoring-and-movement-of-persons-with-exposure.html
- Stehling-Ariza T, Fisher E, Vagi S, et al. Monitoring of persons with risk for exposure to Ebola virus disease—United States, November 3, 2014–March 8, 2015. MMWR Morb Mortal Wkly Rep 2015;64:685–9. PubMed
- Hageman JC, Hazim C, Wilson K, et al. Infection prevention and control for Ebola in health care settings—West Africa and United States. In: CDC response to the 2014–2016 Ebola epidemic—West Africa and United States. MMWR Suppl 2016;65(No. Suppl 3).
- Koonin LM, Jamieson DJ, Jernigan JA, et al. Systems for rapidly detecting and treating persons with Ebola virus disease—United States. MMWR Morb Mortal Wkly Rep 2015;64:222–5. PubMed
- Assistant Secretary for Preparedness and Response. Hospital Preparedness Program (HPP) Ebola Preparedness and Response Activities: development of a regional Ebola and other special pathogen treatment center for HHS Region 9. Washington, DC: US Department of Health and Human Services; 2015. FOA EP-U34–15–002. http://www.grants.gov/view-opportunity.html?oppId=274709
- . CDC. Protecting America from Ebola: CDC’s Ebola response team. http://www.cdc.gov/vhf/ebola/pdf/ebola-response-team.pdf
* 1) Fever (subjective or temperature ≥100.4°F or ≥38.0°C) or symptoms, including severe headache, fatigue, muscle pain, vomiting, diarrhea, abdominal pain, or unexplained hemorrhage AND 2) epidemiologic risk factors including contact with an Ebola patient or patient’s body fluids or travel to a country affected by Ebola within 21 days of symptom onset (http://www.cdc.gov/vhf/ebola/healthcare-us/evaluating-patients/case-definition.html).
† Specialized treatment centers: Emory University Serious Communicable Diseases Unit, Atlanta, Georgia; the National Institutes of Health Clinical Center, Bethesda, Maryland; and the University of Nebraska Biocontainment Unit, Omaha, Nebraska.
§ Initial movement and monitoring guidance was posted on August 22, 2014; the guidance was reviewed and revised as needed throughout the response; the most recent guidance is available at http://www.cdc.gov/vhf/ebola/exposure/monitoring-and-movement-of-persons-with-exposure.html.
¶ DOT Hazardous Materials Regulations (HMR, 49 CFR, Parts 171-180); Ebola virus is classified as a category A infectious substance by the DOT and the United Nations. Category A refers to an infectious substance in a form capable of causing permanent disability or life-threatening or fatal disease in otherwise healthy humans or animals.
 TABLE 1. Key CDC guidance documents for use in domestic preparedness and response to the Ebola epidemic in West Africa — United States, 2014–2016
TABLE 1. Key CDC guidance documents for use in domestic preparedness and response to the Ebola epidemic in West Africa — United States, 2014–2016
| Category | Document |
|---|---|
| Public health preparedness and response | Case Definition for Ebola Virus Disease (EVD): http://www.cdc.gov/vhf/ebola/healthcare-us/evaluating-patients/case-definition.html Interim U.S. Guidance for Monitoring and Movement of Persons with Potential Ebola Virus Exposure: http://www.cdc.gov/vhf/ebola/exposure/monitoring-and-movement-of-persons-with-exposure.html |
| Hospital preparedness | Preparing for Ebola—a Tiered Approach (includes Preparing Frontline Healthcare Facilities; Preparing Ebola Assessment Hospitals; Preparedness Checklists): http://www.cdc.gov/vhf/ebola/healthcare-us/preparing/index.html Infection Prevention and Control Recommendations for Hospitalized Patients Under Investigation (PUIs) for Ebola Virus Disease (EVD) in U.S. Hospitals: http://www.cdc.gov/vhf/ebola/healthcare-us/hospitals/infection-control.html |
| Clinical guidance | Guidance for U.S. Laboratories for Managing and Testing Routine Clinical Specimens When There Is a Concern About Ebola Virus Disease: http://www.cdc.gov/vhf/ebola/healthcare-us/laboratories/safe-specimen-management.html Ebola Virus Disease (EVD) Information for Clinicians in U.S. Healthcare Settings: http://www.cdc.gov/vhf/ebola/healthcare-us/preparing/clinicians.html Guidance on Personal Protective Equipment (PPE) to Be Used by Healthcare Workers During Management of Patients with Confirmed Ebola or Persons Under Investigation (PUIs) for Ebola Who Are Clinically Unstable or Have Bleeding, Vomiting, or Diarrhea in U.S. Hospitals, Including Procedures for Donning and Doffing PPE: http://www.cdc.gov/vhf/ebola/healthcare-us/ppe/guidance.html For U.S. Healthcare Settings: Donning and Doffing Personal Protective Equipment (PPE) for Evaluating Persons Under Investigation (PUIs) for Ebola Who Are Clinically Stable and Do Not Have Bleeding, Vomiting, or Diarrhea: http://www.cdc.gov/vhf/ebola/healthcare-us/ppe/guidance-clinically-stable-puis.html Interim Guidance for Management of Survivors of Ebola Virus Disease in U.S. Healthcare Settings: http://www.cdc.gov/vhf/ebola/healthcare-us/evaluating-patients/guidance-for-management-of-survivors-ebola.html |
| Laboratory guidance | Guidance for U.S. Laboratories for Managing and Testing Routine Clinical Specimens When There Is a Concern About Ebola Virus Disease: http://www.cdc.gov/vhf/ebola/healthcare-us/laboratories/safe-specimen-management.html Collection, Transport, and Submission of Specimens for Ebola Virus Testing: http://www.cdc.gov/vhf/ebola/healthcare-us/laboratories/specimens.html |
| Infection control and waste management | Interim Guidance for Environmental Infection Control in Hospitals for Ebola Virus: http://www.cdc.gov/vhf/ebola/healthcare-us/cleaning/hospitals.html Ebola Waste Management: http://www.cdc.gov/vhf/ebola/healthcare-us/cleaning/waste-management.html Procedures for Safe Handling and Management of Ebola-Associated Waste: http://www.cdc.gov/vhf/ebola/healthcare-us/cleaning/handling-waste.html Interim Guidance for U.S. Residence Decontamination for Ebola and Removal of Contaminated Waste: http://www.cdc.gov/vhf/ebola/prevention/cleaning-us-homes.html Interim Guidance for Ebola Virus Cleaning, Disinfection, and Waste Disposal in Commercial Passenger Aircraft: http://www.cdc.gov/vhf/ebola/prevention/cleaning-commercial-passenger-aircraft.html Interim Guidance for Managers and Workers Handling Untreated Sewage from Individuals with Ebola in the United States: http://www.cdc.gov/vhf/ebola/prevention/handling-sewage.html Guidance for Safe Handling of Human Remains of Ebola Patients in U.S. Hospitals and Mortuaries: http://www.cdc.gov/vhf/ebola/healthcare-us/hospitals/handling-human-remains.html Guidance on Air Medical Transport for Patients with Ebola Virus Disease: http://www.cdc.gov/vhf/ebola/healthcare-us/emergency-services/air-medical-transport.html Interim Guidance for Emergency Medical Services Systems and 9-1-1 Public Safety Answering Points for Management of Patients Under Investigation for Ebola Virus Disease in the United States: http://www.cdc.gov/vhf/ebola/healthcare-us/emergency-services/ems-systems.html |
| Patient transportation | Guidance on Air Medical Transport for Patients with Ebola Virus Disease: http://www.cdc.gov/vhf/ebola/healthcare-us/emergency-services/air-medical-transport.html Interim Guidance for Emergency Medical Services Systems and 9-1-1 Public Safety Answering Points for Management of Patients Under Investigation for Ebola Virus Disease in the United States: http://www.cdc.gov/vhf/ebola/healthcare-us/emergency-services/ems-systems.html Guidance for Developing a Plan for Interfacility Transport of Persons Under Investigation or Confirmed Patients with Ebola Virus Disease in the United States: http://www.cdc.gov/vhf/ebola/healthcare-us/emergency-services/interfacility-transport.html |
Abbreviation: Ebola = Ebola virus disease.
 TABLE 2. Abbreviated timeline of the domestic response to the Ebola epidemic in West Africa — United States, 2014–2016
TABLE 2. Abbreviated timeline of the domestic response to the Ebola epidemic in West Africa — United States, 2014–2016
| Date | Event |
|---|---|
| 2014 | |
| July 9 | CDC EOC is activated to support Ebola response. |
| August 2 | HCW with Ebola diagnosed in West Africa is admitted to Emory University Hospital in Atlanta, Georgia. |
| August 7 | First version of CDC Interim U.S. Guidance for Monitoring and Movement of Persons with Potential Ebola Virus Exposure posted. |
| September 20 | Businessman from Liberia arrives in Dallas, Texas, after negative fever screening on departure from Liberia and entry into United States. |
| September 25 | After 1 day of symptoms, Liberian businessman seeks care at Texas Health Presbyterian Hospital in Dallas, is treated for presumed sinusitis and discharged. |
| September 28 | Liberian businessman remains ill, is admitted to hospital. |
| September 30 | Ebola diagnosed in Liberian businessman; he becomes first person with Ebola diagnosed in the United States. CDC and Texas health officials begin contact tracing and identify 48 total possible or confirmed contacts of the U.S. index patient before his isolation at the hospital; active monitoring of these contacts begins. |
| October 8 | First person with Ebola diagnosed in the United States dies. |
| October 11–16 | CDC and CBP begin enhanced entry risk assessment and management at five U.S. airports (JFK: October 11; EWR, IAD, ORD, and ATL: October 16) that receive approximately 94% of travelers from Guinea, Liberia, and Sierra Leone. |
| October 11 | A nurse (nurse 1) who provided care for the Ebola patient in Dallas develops fever, seeks care in an emergency department; Ebola is diagnosed. |
| October 12 | CDC and Texas health officials begin active monitoring of household contact of nurse 1. CDC begins active monitoring of 76 hospital workers who treated first patient with Ebola diagnosed in the United States. Active monitoring begins for all 147 HCW contacts of any of the Ebola patients, irrespective of PPE use; monitoring continues until 21 days from their last exposure. |
| October 14 | A second nurse (nurse 2) who provided care for the Ebola patient in Dallas develops fever and is hospitalized. CDC, Texas, and Ohio health officials begin contact tracing of contacts of nurse 2 and active monitoring of three household contacts. |
| October 15 | Ebola is diagnosed in nurse 2, who is transferred to Emory University Hospital in Atlanta. CDC notifies a domestic airline that a passenger (nurse 2) who traveled from Cleveland, Ohio, to Dallas on October 13 tested positive for EBOV. |
| October 16 | Nurse 1 is transferred from Texas Health Presbyterian Hospital to the National Institutes of Health hospital in Bethesda, Maryland. |
| October 19 | CDC REP teams begin visits to U.S. hospitals to provide technical assistance. |
| October 20 | CDC revises guidance on PPE for U.S. HCWs caring for Ebola patients. |
| October 21 | CBP announces that all travelers from Guinea, Liberia, and Sierra Leone will be routed to one of five participating U.S. airports for enhanced entry risk assessment and management. |
| October 23 | New York City Department of Health and Mental Hygiene diagnoses Ebola in an HCW (HCW 1) who had returned to New York City from Guinea; patient is isolated at Bellevue Hospital. |
| October 24 | CDC and New York City health officials begin contact tracing of HCW 1’s contacts before isolation at the hospital. An asymptomatic HCW (HCW 2) who returned to the United States after treating patients in Sierra Leone is isolated by New Jersey officials at a nearby hospital. |
| October 27 | CDC issues revised Interim U.S. Guidance for Monitoring and Movement of Persons with Potential Ebola Virus Exposure. Active postarrival monitoring begins for travelers from Guinea, Liberia, and Sierra Leone. HCW 2 is released from quarantine and drives from New Jersey to Maine. |
| October 28 | Nurse 2 is discharged from Emory hospital after being declared Ebola virus free. |
| October 29 | Monitoring is completed for 47 of 48 initial contacts of Dallas index patient. |
| October 30 | Maine judge issues a 1-day court-ordered restriction of HCW 2’s movements. |
| October 31 | Active monitoring is completed for passengers and crew on October 10 airline flight (Dallas to Cleveland) on which nurse 2 traveled. |
| November 3 | HCW 2 agrees to daily monitoring by Maine state health department. Active monitoring is completed for passengers and crew on October 13 airline flight (Cleveland to Dallas) on which nurse 2 traveled. |
| November 7 | Active monitoring is completed for all 177 contacts of Ebola patient in Dallas and nurses 1 and 2 (some persons were contacts of more than one patient) after completing 21 days of monitoring; Ebola did not develop in any contacts. |
| November 10 | Active monitoring of HCW 2 is discontinued. |
| November 11 | HCW 1 is discharged from Bellevue Hospital in New York City. |
| November 17 | Travelers from Mali are routed to one of five U.S. airports for enhanced entry risk assessment and management. |
| December 2 | Guidance is released for tiered approach to health care facility preparedness. |
| 2015 | |
| May 9 | WHO declares end of the Ebola epidemic in Liberia. |
| June 29 | New cases of Ebola are reported in Liberia. |
| September 3 | WHO declares Liberia free of EBOV transmission for the second time. |
| November 7 | WHO declares Sierra Leone free of EBOV transmission. |
| November 19 | New cases of Ebola are reported in Liberia. |
| December 29 | WHO declares Guinea free of EBOV transmission. |
| 2016 | |
| January 14 | WHO declares Liberia free of EBOV transmission for the third time. |
| February 19 | U.S. government discontinues enhanced entry screening procedures and airline routing for Ebola. CDC retires the Interim U.S. Guidance for Monitoring and Movement of Persons with Potential Ebola Virus Exposure. |
| March 17 | New cases of Ebola are reported in Guinea. |
| April 1–4 | New cases of Ebola are reported in Liberia. |
Abbreviations: ATL = Hartsfield–Jackson Atlanta International Airport; CBP = Customs and Border Protection, U.S. Department of Homeland Security; Ebola = Ebola virus disease; EBOV = Ebola virus; EOC = Emergency Operations Center; EWR = Newark Liberty International Airport; HCW = health care worker; IAD = Washington Dulles International Airport; JFK = John F. Kennedy International Airport (New York City); ORD = Chicago O’Hare International Airport; PPE = personal protective equipment; REP = Rapid Ebola Preparedness; WHO = World Health Organization.
 FIGURE. Tiered approach for U.S. hospital and health care facility* preparedness for Ebola
FIGURE. Tiered approach for U.S. hospital and health care facility* preparedness for Ebola
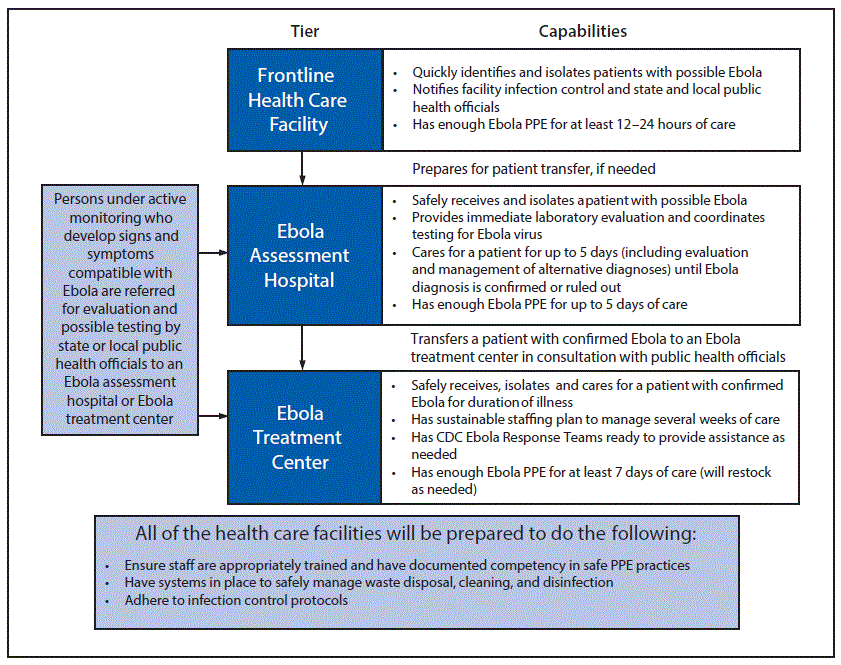
Abbreviations: Ebola = Ebola virus disease; PPE = personal protective equipment.
* Ebola treatment center includes regional treatment centers for Ebola and other special pathogens.
Suggested citation for this article: Van Beneden CA, Pietz H, Kirkcaldy RD, et al. Early Identification and Prevention of the Spread of Ebola — United States. MMWR Suppl 2016;65(Suppl-3):75–84. DOI: http://dx.doi.org/10.15585/mmwr.su6503a11.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (https://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.
- Page last reviewed: July 17, 2017
- Page last updated: July 17, 2017
- Content source:


 ShareCompartir
ShareCompartir