Vaccination Strategies
In a smallpox emergency, the first-line vaccination strategy will be ring vaccination. This strategy was used to successfully eradicate smallpox in the late 20th century. If there are not enough personnel to trace and vaccinate all contacts quickly enough, public health authorities may augment ring vaccination with a mass vaccination (community-wide) strategy.
CDC has stockpiled enough smallpox vaccine to vaccinate every person in the United States. In a smallpox emergency, CDC will coordinate with the Medical Countermeasures (MCM) coordinator or the preparedness office in the state or territorial health department. The MCM coordinator will allocate vaccine to local areas, depending upon the circumstances of the emergency.
Key Points for Vaccination Plans
There are three different smallpox vaccines that each require distinct implementation considerations in a smallpox emergency vaccination strategy: ACAM2000®, APSV, and Imvamune. The following highlighted considerations are not comprehensive. Full documentation of vaccine-related clinical information and implementation considerations are found in the Vaccination section for Clinicians. For more information, refer to the Clinical Guidance for Smallpox Vaccine Use in a Postevent Vaccination Program.
ACAM2000® and APSV Considerations
ACAM2000® is licensed by the U.S. Food and Drug Administration (FDA) and will be used if there is ever a smallpox emergency. APSV has a similar safety profile to ACAM2000® and is also held by the Strategic National Stockpile for use in a smallpox emergency. It will be used under an Emergency Use Authorization (EUA) or Investigational New Drug application (IND) if ACAM2000® is depleted, is not readily available, or on a case-by-case basis for individuals with an allergy to a component of ACAM2000®.
ACAM2000® and APSV are both administered as a single dose by the percutaneous route using the multiple puncture technique. Medical and public health personnel will likely not be familiar with this administration technique, so both advance and just-in-time training will be needed. Training for this unfamiliar method of vaccination may take a couple of hours, even among experienced medical personnel.
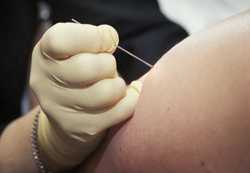
Medical personnel with gloved hand administering smallpox vaccine with a bifurcated needle
ACAM2000® and APSV are live virus vaccines containing vaccinia virus. Those vaccinated with either of these vaccines who do not properly care for their vaccination site may spread the vaccinia virus to other parts of their body or to other people, and therefore require vigilant vaccination site care from the day of vaccination until the scab falls off, which usually happens about 14 to 21 days later.
Educate vaccinees on how to care for their vaccination sites properly, what a “take” should look like, and how to seek care for and report any serious adverse events to vaccination. Full immunity is achieved after the confirmation of a “take” 6 to 8 days after vaccination.
Imvamune Considerations
Imvamune is an investigational vaccine. In a smallpox emergency, Imvamune would be made available for use under an Emergency Use Authorization (EUA) and according to clinical recommendations for persons of all ages with atopic dermatitis or certain immune deficiencies, such as HIV. Supplies of Imvamune may be limited in situations requiring widespread vaccination. When planning how to provide vaccinations, consider ways to work with medical facilities that may be more effective at reaching persons eligible for Imvamune, such as HIV or dermatology clinics.
Unlike ACAM2000® and APSV, Imvamune is administered subcutaneously as two doses separated by 4 weeks (one dose at week 0 and a second dose at week 4) for primary vaccinees (individuals who have never been vaccinated against smallpox or do not recall receiving a smallpox vaccine in the past). Individuals previously vaccinated against smallpox receive one dose.
Full immunity may not develop until 2 weeks after the second dose (for primary vaccinees). This delay may leave individuals not fully protected for up to 6 weeks after their initial vaccination. Individuals receiving Imvamune will need to be especially vigilant to avoid exposure to smallpox patients and the vaccination sites of those vaccinated with ACAM2000® or APSV during the 6 weeks after receiving the first dose and before developing immunity. Smallpox vaccination plans should include ways to ensure these vaccinees return for the second dose.
Imvamune does not produce a noticeable “take” like ACAM2000® or APSV. While this means there is no chance of secondary vaccinia transmission with this vaccine, the lack of a noticeable “take” makes it difficult to know whether or not the vaccinee has developed immunity.
Cold Chain Management
SNS will ship vaccines in a manner consistent with maintaining the cold chain. ACAM2000® or APSV are shipped refrigerated. Imvamune may be shipped and stored either frozen or refrigerated. Cold chain management plans should include the possibility of maintaining the cold chain of vaccines at two different temperatures.
Vaccine Supply Tracking
Keep accurate counts of vaccine doses given and lost during an outbreak. CDC and state public health departments will use the information reported by local public health departments to manage the supply and distribution of vaccine during an emergency.
Screening Considerations
Because of the high case-fatality rate and severity of smallpox, no clear absolute contraindications exist for the use of smallpox vaccines for those exposed to smallpox virus or at high risk for smallpox infection. Plan for screening potential vaccinees thoroughly, using established criteria. Most people will meet the criteria for vaccination with ACAM2000® or APSV. Clinicians should be available to consult on individuals with a health status that may require using Imvamune instead of ACAM2000® or APSV.
Staff Vaccination
Staff should be vaccinated, but there is no waiting period between when they receive their vaccination and when they can start administering vaccinations or working in vaccination clinics. To prevent the spread of vaccinia virus, any clinical staff who provide vaccinations or care to patients should follow enhanced vaccination site care methods.
Vaccinee Follow-up
For those vaccinated with ACAM2000® or APSV: Identify a time and place for vaccinees to have their vaccination site evaluated. Use the number of people vaccinated and the days on which they were vaccinated to determine the number and timing of staff needed for the follow-up evaluations. Include plans for revaccinating those with non-takes. Follow-up on vaccinees who do not come in for a take evaluation.
For those vaccinated with Imvamune: Identify a time and place for primary vaccinees to receive their second dose of Imvamune, 4 weeks after the first dose. Include plans for following-up on vaccinees who do not come in for their second dose of vaccine.
Adverse Event Monitoring
While rare, adverse events may happen, especially within a large population of new vaccinees. Give vaccinees information about who to contact or where they can go for medical care if they suspect they are having an adverse event to the vaccination. Consider how to monitor and report these adverse events to public health authorities quickly in overall surveillance plans. Smallpox response plans should also ensure that all adverse events are reported to the Vaccine Adverse Events Reporting System (VAERS).
Countermeasures Injury Compensation Program
The Countermeasures Injury Compensation Program (CICP) is a federal program created to help pay for related costs of medical care and other specific expenses for eligible people seriously injured by the administration or use of certain medical countermeasures. Medical countermeasures may include vaccines, medications, devices, or other items used to prevent, diagnose, or treat the public during a current, or potential future, public health emergency or security threat. For more information, visit the CICP website or call: 1-855-266-2427. In an emergency, provide information about this program to vaccinees.
Resource
Clinical Guidance for Smallpox Vaccine Use in a Postevent Vaccination Program Source: MMWR. 2015, 64(RR02);1-26.
- Page last reviewed: January 6, 2017
- Page last updated: January 6, 2017
- Content source:


 ShareCompartir
ShareCompartir