Packaging and Transporting Infectious Substances
Category A Infectious Substance
Package, label, and ship high-risk specimens as a Category A infectious substance affecting humans (UN 2814) in accordance with the U.S. Department of Transportation’s Hazardous Materials Regulations and the International Air Transport Association Dangerous Goods Regulations.
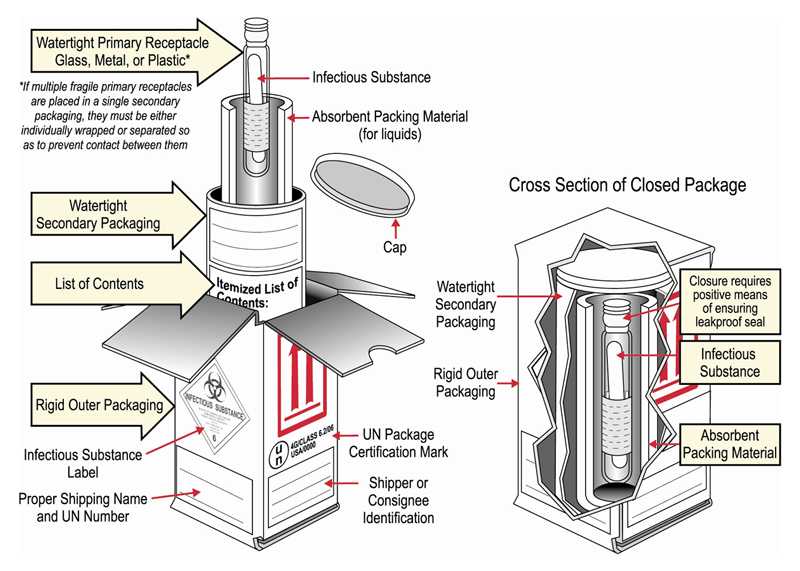
Packing and shipping Category A clinical specimens diagram
All persons packing and shipping infectious materials must be trained and certified every two years in compliance with the Department of Transportation or the International Air Transport Association.
- Triple pack all specimens in:
- Leakproof primary receptacle; multiple primary receptacles should be individually wrapped or separated
- Leakproof secondary receptacle, and
- Rigid outer packaging
- If specimen is a liquid, place absorbent material between the primary and secondary receptacle.
- Place a list of contents and paperwork between the secondary receptacle and outer packaging.
- Label outer packaging with:
- Infectious substance (diamond shaped label)
- Proper shipping name and UN 2814 certification mark
- Shipper and consignee identification (name, address, and telephone)
- Package orientation arrows if primary receptacle exceeds 50 mL or more
- Complete and submit CDC Form 50.34 and the Poxvirus Human Specimen Submission Form (provided at time of consultation) with the shipment.
- Ship to the following address:
- Centers for Disease Control and Prevention
Attn: STAT Lab / Poxvirus Program
1600 Clifton Road NE
Atlanta, GA 30329
Phone: 404.639.4129
- Centers for Disease Control and Prevention
Category B Infectious Substance
Package, label, and ship low- or moderate-risk specimens as a Category B infectious substance (UN 3373) in accordance with the U.S. Department of Transportation’s Hazardous Materials Regulations and the International Air Transport Association Dangerous Goods Regulations.
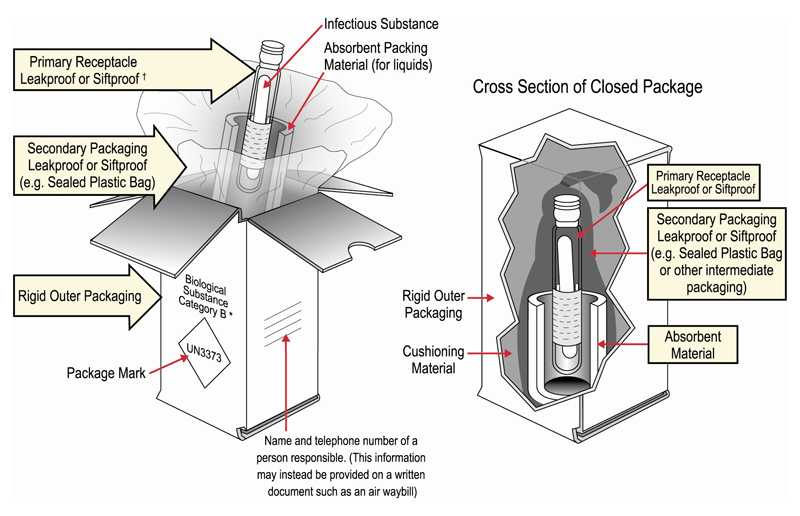
Packing and shipping Category B clinical specimens diagram
- Triple pack the specimens in:
- Leakproof primary receptacle; multiple primary receptacles should be individually wrapped or separated
- Leakproof secondary receptacle
- Rigid or strong outer packaging
- If specimen is a liquid, place absorbent material between the primary and secondary receptacle.
- Place a list of contents and paperwork between the secondary receptacle and outer packaging.
- Label outer package with:
- Proper shipping name and UN 3373 certification mark
- Shipper and consignee identification (name, address, and telephone)
- Package orientation arrows if primary receptacle exceeds 50 mL or more
- Complete and submit CDC Form 50.34 and the Poxvirus Human Specimen Submission Form (provided at time of consultation) with the shipment.
- Ship to the following address:
- Centers for Disease Control and Prevention
Attn: STAT Lab / Poxvirus Program
1600 Clifton Road NE
Atlanta, GA 30329
Phone: 404.639.4129
- Centers for Disease Control and Prevention
- Page last reviewed: December 1, 2016
- Page last updated: December 1, 2016
- Content source:


 ShareCompartir
ShareCompartir