Polysaccharide from Sucrose Test
Principle
Some bacterial species produce a starch-like polysaccharide from sucrose which stains dark blue-purple to black with iodine. Among the Neisseria spp., N. perflava biovar perflava, N. mucosa, N. sicca, N. flavescens, and N. polysaccharea produce polysaccharide from sucrose. This test is invaluable for differentiating between strains of N. meningitidis (polysaccharide-negative) and N. polysaccharea (polysaccharide-positive); as many as 25% of organisms identified as nontypable N. meningitidis strains were found to be N. polysaccharea when tested with the polysaccharide production test.
Traditionally, polysaccharide production was detected on a starch-free medium containing 5% (w/v) sucrose. However, 5% sucrose is inhibitory for some strains ; polysaccharide may be detected on a starch-free medium containing 1% sucrose. Strains are inoculated onto the medium to give either well-isolated colonies; polysaccharide may not be detected in confluent growth. Although some strains, e.g., N. gonorrhoeae, may not grow on the medium, a polysaccharide test may be performed if a heavy inoculum is deposited on the medium. The test does not required incubation in an a carbon dioxide-supplemented (5%) atmosphere; carbon dioxide may be provided to support growth of the strain.
After incubation for 48 h. at 35-36.5C in a incubator with or without supplemental carbon dioxide, the starch-like polysaccharide, if produced, is detected by adding a drop of iodine solution (Gram's iodine or Lugol's iodine [Gram's iodine diluted 1:4]) to the growth; the polysaccharide stains dark blue-purple to black (Figure 1). The growth of strains which do not produce polysaccharide may acquire a light brown color due to the iodine solution but, otherwise, shows no darkening (Figure 1).
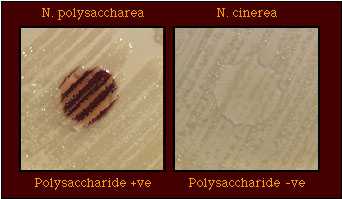
Figure 1. Production of Polysaccharide from Sucrose by N. polysaccharea, but Not by N. cinerea.
N. polysaccharea (left) produces polysaccharide from sucrose; N. cinerea (right) does not produce polysaccharide from sucrose.
Although the traditional method for detection of polysaccharide recommends incubation for 48 h., polysaccharide may be detected after incubation for 24 h. and up to 3-4 days. However, strains that produce the polysaccharide may also degrade it through the action of amylosucrase. Thus, the reaction may be weaker, or absent, if strains are incubated longer than 48 h.; polysaccharide may not be detected if strains are incubated for 5 days. The stain reaction may fade with time but will immediately reappear if more iodine solution is added to the growth.
It is not possible to detect polysaccharide in the sucrose-containing medium of rapid acid detection tests such as QuadFERM+.
Specimen Requirements
Optimum specimen:
A pure culture of a suspect gram-negative, oxidase-positive diplococcus on chocolate agar incubated in a carbon dioxide-supplemented atmosphere at 35C to 36.5C for 18 to 24 h.
Unacceptable specimen:
Cultures of isolates on chocolate agar incubated in a carbon dioxide-supplemented atmosphere at 35C to 36.5C for more than 24 h.
Compromising factors affecting test results:
- The test should be performed after 24 to 48 h. incubation for the best results. Polysaccharide may be metabolized by the organism and may not be detectable after incubation for 3 to 5 days.
- Commercial Gram's iodine, sold as a Gram stain reagent, may not react with the polysaccharide. Iodine used to detect the polysaccharide may have to be made from scratch.
Stability of specimen:
- The polysaccharide may be detected after 24 to 48 h. incubation, after which it may be metabolized by the producing organisms. Polysaccharide is stable within the optimum incubation times.
- Polysaccharide is stable when plates/tube medium are removed from the incubator. The reaction will fade with time, but can be reproduced by the addition of more iodine reagent.
Reagents & Equipment
Medium: Polysaccharide medium (Tryptic soy agar containing 1% sucrose)
Tryptic soy agar (Difco), 40.0 g
Distilled (ETF; Endotoxin-free) water, 1000.0 ml
Sucrose, Reagent grade
- Suspend tryptic soy agar in ETF water.
- Autoclave at 121C for 15 min. Cool to 50C.
- Prepare 10% sucrose (Reagent grade) solution and filter sterilize using a 0.45 micron filter.
- Aseptically add sucrose solution to agar to give a final concentration of 1% (W/V).
- Dispense 20 to 25 ml. volumes in 100 mm Petri dishes.
Store medium at 4C to 10C (refrigerated) until used. Prewarm the medium to room temperature before inoculation.
Reagent: Lugol's iodine solution (Gram's iodine solution diluted 1:4)
Note: Iodine solutions provided with commercially available Gram strain kit reagents may not react with polysaccharide to give a positive reaction with strains known to be positive in this test. It may be necessary to make the iodine solution from the original formulation for Gram's iodine. Alternatively, Gram's iodine may be used.
Store iodine solution (Gram's iodine) at room temperature (15C to 30C) in the dark (wrapped in aluminum foil). Reagent may be used until quality assurance tests fail.
Quality Control/Test Procedure
QC strains:
- Polysaccharide-positive control: Neisseria polysaccharea, ATCC 43768
Polysaccharide-negative control: Neisseria cinerea, CDC 10,256
QC strains are stored at -70C in a solution of tryptic soy broth containing 20% glycerol. Reactions of control strains should be confirmed at the time frozen stocks are prepared. QC strains may be stored at -70C for 2 years.
Procedure
Quality Control (QC) strains are tested in the same manner as test strains. QC strains should be subcultured at least once after the initial culture from the frozen specimen before the test is performed.
- Thaw vials of quality control strains stored at -70C.
- Streak onto plates of chocolate agar or supplemented GC base medium to obtain isolated colonies and incubate at 35C to 36.5C in a carbon dioxide-enriched atmosphere for 48 h.
Inoculate polysaccharide medium for isolated colonies or confluent growth with a sterile swab or inoculating loop and incubate medium at 35C-36.5C in a carbon dioxide-enriched atmosphere for 18h. to 24 h.
Note: Strains of N. gonorrhoeae and some other Neisseria spp. may not grow well on this medium. Thus, reaction may be dependent on preformed enzyme; plate should be inoculated heavily and confluently.
After incubation add, with Pasteur pipettes, one drop of Gram's iodine to growth on plate. The growth of strains that produce polysaccharide will immediately turn dark brown to purple or black.
If the growth turns brown, purple, or black when the iodine solution is poured onto the colonies, the reaction is positive (Figure 1): the strain is "Polysaccharide-positive."
If the growth does not change color (other than a light brown color due only to the color of the iodine reagent, the reaction is negative (Figure 1): The strain is "Polysaccharide-negative."
- Read and record results.
Quality Control Schedule:
- A polysaccharide production QC test is performed each day that clinical isolates are tested.
Problems & Solutions
The reaction of a polysaccharide-positive strain may fade with time. The reaction may be developed again by adding another drop of iodine to the growth.
False-negative reactions may be obtained if inoculated medium is incubated longer than 48 h. because amylosucrase (produced by polysaccharide-positive organisms) degrades the polysaccharide that has been produced.
The polysaccharide may not be detected with commercially available Gram's iodine reagent.
Limitations of Test
If the test is performed properly and quality control strains give appropriate results, there should be no limitations to this test. Care must be taken to ensure that all components of the test are performed properly.
No identification of genus or species may be made on the basis of this test alone. The polysaccharide test aids differentiation among strains that may exhibit identical acid production patterns or produce hydroxyprolylaminopeptidase-positive or gamma-glutamylaminopeptidase-positive reactions in enzyme substrate tests.
Results, Interpretation, & Reporting
Isolates may be reported as:
"Polysaccharide-positive" if the growth of an isolate turns dark brown, purple, or black after the addition of Gram's iodine solution.
"Polysaccharide-negative" if the color of the growth does not change color other than the color contributed by the iodine reagent.
Bibliography
Berger U. Polysaccharidbildung durch saprophytische Neisserien. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg Abt 1 Orig. 1961;183:345-348.
Knapp JS. Historical perspectives and identification of Neisseria and related species. 1988. Clin Microbiol Rev 1988;1:415-431.
Boquette MT, Marcos C, Saez-Nieto JA. Characterization of Neisseria polysaccharea sp. nov. (Riou, 1983) in previously identified noncapsular strains of Neisseria meningitidis. J Clin Microbiol 1986;23:973-975.
Knapp JS, Hook EW III. Prevalence and presistence of Neisseria cinerea and other Neisseria spp. in adults. J Clin Microbiol 1988;26:896-900.
- Page last reviewed: December 10, 2013
- Page last updated: October 17, 2008
- Content source:


 ShareCompartir
ShareCompartir