Chapter 8: Identification and Characterization of Streptococcus pneumoniae
Printer friendly version [14 pages]
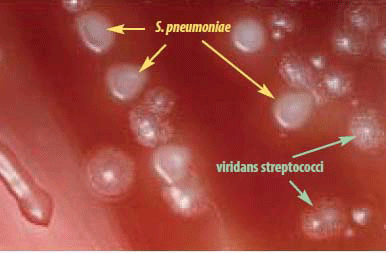
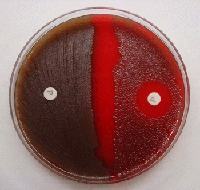
S. pneumoniae may occur intracellularly or extracellularly as gram-positive lanceolate diplococci, but can also occur as single cocci or in short chains of cocci. S. pneumoniae is a fastidious bacterium, growing best at 35-37°C with ~5% CO2 (or in a candle-jar). It is usually cultured on media that contain blood, but can also grow on a chocolate agar plate (CAP). On a blood agar plate (BAP), colonies of S. pneumoniae appear as small, grey, moist (sometimes mucoidal), colonies and characteristically produce a zone of alpha-hemolysis (green) (Figure 1). The alpha-hemolytic property differentiates this organism from many species, but not from the commensal alpha-hemolytic (viridans) streptococci. Differentiating pneumococci from viridans streptococci is difficult as young pneumococcal colonies appear raised, similar to viridans streptococci. However, once the pneumococcal culture ages 24-48 hours, the colonies become flattened, and the central portion becomes depressed, which does not occur with viridans streptococci (Figure 2). A microscope (30-50X) or a 3X hand lens can also be a useful tool in differentiating pneumococci from viridans streptococci. Prior to identification and characterization testing procedures, isolates should always be inspected for purity of growth and a single colony should be re-streaked, when necessary, to obtain a pure culture. For the following identification and characterization procedures, it is essential to test alpha-hemolytic colonies that are less than a day old, typically grown overnight at 35-37°C with ~5% CO2 (or in a candle-jar).
The following specialized tests are used to identify colonies on a BAP that resemble pneumococci (Figure 3). S. pneumoniae can be identified using Gram stain, catalase, and optochin tests simultaneously, with bile solubility as a confirmatory test. If these tests indicate that the isolate is S. pneumoniae, serological tests to identify the serotype can be performed. This sequence of testing is an efficient way to save costly serotyping reagents and time. Additional methods for identification and characterization of S. pneumoniae using molecular tools are described in Chapter 10: PCR Methods and Chapter 12: Molecular Methods. See additional protocols used for streptococcal species identification and updates to existing methods.
Biosafety Level 2 (BSL-2) practices are required for work involving isolates of S. pneumoniae, as this organism presents a potential hazard to laboratory personnel and the surrounding working environment. Please refer to Chapter 4: Biosafety in order to follow the guidelines that have been established for laboratorians working in BSL-2 facilities as many of the tests described in this chapter require opening plates with live cultures and are often performed outside of a biosafety cabinet (BSC).
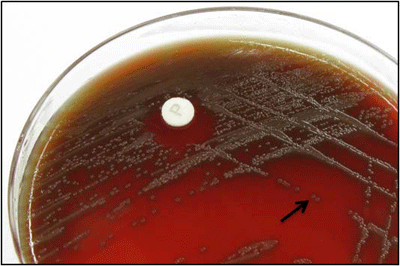
Figure 1. S. pneumoniae colonies with a surrounding green zone of alpha-hemolysis (black arrow) on a BAP
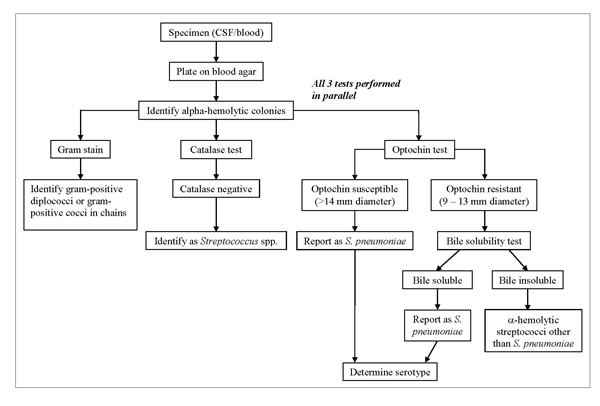
Figure 3. Flow chart for identification and characterization of a S. pneumoniae isolate...view larger
-
Catalase test
Catalase is the enzyme that breaks down hydrogen peroxide (H2O2) into H2O and O2. The oxygen is given off as bubbles in the liquid. The catalase test is primarily used to differentiate between gram-positive cocci. Members of the genus Staphylococcus are catalase-positive, and members of the genera Streptococcus and Enterococcus are catalase-negative.
- Performing the catalase test
- Grow the isolate(s) to be tested for 18-24 hours on a BAP at 35-37°C with ~5% CO2 (or in a candle-jar).
- From overnight growth on the BAP, use a disposable loop to carefully remove a colony and place it on a glass slide.
- Do not transfer any of the blood agar to the slide as erythrocytes in the blood agar will cause a false-positive reaction.
- Add 1.0 ml of 3% H2O2 to the slide and mix with the bacteria.
- H2O2 can be obtained from a commercial drug store.
- After initially opening, store H2O2 at 4°C in a tightly closed bottle as it will slowly lose potency once opened.
- Observe the bacterial suspension on the slide immediately for vigorous bubbling.
- It is essential to use a known positive and negative quality control (QC) strain. A Staphylococcus spp. strain can be used for a positive control and a known S. pneumoniae strain or any other streptococcal spp., i.e., S. pyogenes can be used for a negative control.
- Reading the catalase test results
- The absence of bubbling from a transferred colony indicates a negative test.
- Any bubbling from a transferred colony indicates a positive test (Figure 4).
- Troubleshooting
- False positives will result from transfer of red blood cells so take care when picking colonies from the BAP for this test.
- Quality control
- It is essential to use a known positive and negative QC strain as described in the procedure. Opened bottles should be checked against a known catalase positive organism every 6 months.
- Performing the catalase test
-
Optochin test
S. pneumoniae strains are sensitive to the chemical optochin (ethylhydrocupreine hydrochloride). Optochin sensitivity allows for the presumptive identification of alpha-hemolytic streptococci as S. pneumoniae, although some pneumococcal strains are optochin-resistant. Other alpha-hemolytic streptococcal species are optochin-resistant.
-
Performing the optochin test
Optochin (P) disks (6 mm, 5 µg) can be obtained from a commercial vendor. Optochin disks are often called "P disks" and many commercial versions are labeled with a capital "P". If a commercial source of P disks is not available, a 1:4000 solution of ethylhydrocupreine hydrochloride can be applied to sterile 6 mm filter paper disks.
- Grow the strain(s) to be tested for 18-24 hours on a BAP at 35-37°C with ~5% CO2 (or in a candle-jar).
- Use a disposable loop to remove an isolated colony from the overnight culture on the BAP and streak onto one half of a BAP.
- Two different isolates can be tested on the same plate, but care must be taken to ensure that the cultures do not overlap.
- Place a P disk within the streaked area of the plate and incubate the BAP overnight at 35-37°C with ~5% CO2 (or in a candle-jar).
- Observe the growth on the BAP near the P disk and measure the zone of inhibition, if applicable.
- Reading the optochin test results
- Using a 6 mm, 5 µg disk, a zone of inhibition of 14 mm or greater indicates sensitivity and allows for presumptive identification of pneumococci (Figure 5).
- Zones of inhibition should be measured from the top surface of the plate with the top removed.
- Use either calipers or a ruler with a handle attached for these measurements. Measure the diameter of the zone holding the ruler over the center of the surface of the disk when measuring the zone of inhibition. In the case of an isolate completely resistant to optochin, the diameter of the disk (6 mm) should be recorded.
- Troubleshooting
- A smaller zone of inhibition (< 14 mm) or no zone of inhibition indicates that the bile solubility test is required. It is important to remember that pneumococci are sometimes optochin-resistant.
- Quality control
- Each new lot of optochin disks should be tested with positive and negative controls. The growth of S. pneumoniae strain ATCC 49619 is inhibited by optochin and growth of S. mitis strain ATCC 49456 is not inhibited by optochin.
-
-
Bile solubility test
The bile (sodium deoxycholate) solubility test distinguishes S. pneumoniae from all other alpha-hemolytic streptococci. S. pneumoniae is bile soluble whereas all other alpha-hemolytic streptococci are bile resistant. Sodium deoxycholate (2% in water) will lyse the pneumococcal cell wall.
- Preparation of 2% sodium deoxycholate (bile salt) solution
- Dissolve 2 g of sodium deoxycholate into 100 ml sterile distilled water.
- Performing the bile solubility test
- Grow the isolate(s) to be tested for 18-24 hours on a BAP at 35-37°C with ~5% CO2 (or in a candle-jar).
- Add bacterial growth from the overnight BAP to 1.0 ml of 0.85% saline to achieve turbidity in the range of a 0.5-1.0 McFarland standard.
- Divide the cell suspension equally into 2 tubes (0.5 ml per tube).
- Add 0.5 ml of 2% sodium deoxycholate (bile salts) to one tube. Add 0.5 ml of 0.85% saline to the other tube. Mix each tube well.
- Incubate the tubes at 35-37°C in CO2.
- Vortex the tubes.
- Observe the tubes for any clearing of turbidity after 10 minutes. Continue to incubate the tubes for up to 2 hours at 35-37°C in CO2 if negative after 10 minutes. Observe again for clearing.
- Reading the bile solubility test results
- A clearing of the turbidity in the bile tube but not in the saline control tube indicates a positive test (Figure 6).
- Troubleshooting
- Partial clearing (partial solubility) is not considered positive for pneumococcal identification. Partially soluble strains that have optochin zones of inhibition of less than 14 mm are not considered pneumococci.
- Quality control
- Each new lot of sodium deoxycholate should be tested with positive and negative QC strains. S. pneumoniae strain ATCC 49619 can be used as a positive control and S. mitis strain ATCC 49456 can be used as a negative control.
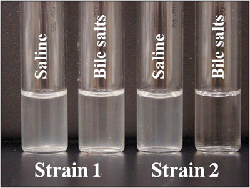
Figure 6. Results of the bile solubility test are shown for two different strains of bacteria. For strain 1, a slight decrease in turbidity is observed in the tube containing the bile salts (2nd from left), but the contents are almost as turbid as the control tube (far left); therefore, strain 1 is not S. pneumoniae. For strain 2, all turbidity in the tube containing the bile salts (far right) has cleared, indicating that the cells have lysed, in contrast to the control tube (2nd from right), which remains turbid; therefore, strain 2 is S. pneumoniae.
- Preparation of 2% sodium deoxycholate (bile salt) solution
-
Commercial test kits for identification
Several commercial identification systems that use slide agglutination tests are available for identification of colony growth from a BAP as S. pneumoniae. These identification tests use suspensions of latex beads with rabbit antibody specific for S. pneumoniae capsular antigens. Visible agglutination occurs when the S. pneumoniae capsular antigen reacts with the antibody-coated latex beads. The manufacturer's instructions should be followed precisely when using these kits. These kits should be regularly subjected to QC using a non-pneumococcal streptococcal species, since they can become cross-reactive with prolonged storage.
-
Determining S. pneumoniae capsular serotypes using serologic methods
Although serotyping of pneumococci is not usually necessary for a clinical response, capsular serotype determination is a critical component of successful pneumococcal disease surveillance efforts. Effective current multivalent vaccines target combinations of key serotypes. Determination of serotype distributions associated with disease in certain regions provides information regarding the potential usefulness of applying existing vaccines and is also critical for assessing vaccine impact.
Serotype distribution can be determined by culture of the organism followed by serological determination of the capsular type by latex agglutination and the quellung reaction. Many laboratories have opted to use simpler and less expensive methods of deducing capsular serotypes through the use of specific PCR reactions (see Chapter 10: PCR Methods and PCR Deduction of Pneumococcal Serotypes for specific PCR protocols).
-
Latex agglutination
The standard quellung reaction test for serotyping pneumococci can be labor-intensive and time-consuming, and requires a certain level of experience to be performed competently. An agglutination method using anti-rabbit IgG-coated latex particles sensitized to pooled and select individual serotype-specific antisera (PCV7 serotypes: 4, 6B, 9V, 14, 18C, 19F, 23F) for serogrouping/serotyping S. pneumoniae has been developed and kits are commercially available. The latex agglutination method is simpler and faster than the quellung reaction, but is only intended for partial serotyping as it can only narrow the identification down to a group or pool of serotypes. Then the quellung reaction should be performed using individual serotype-specific antisera for each serotype in the group or pool to identify the serotype.
Performing latex agglutination testing
- Grow the isolate(s) to be tested for 18-24 hours on a BAP at 35-37°C with ~5% CO2 (or in a candle-jar).
- From overnight growth on a BAP, use a sterile loop to prepare a light to moderate cell suspension (approximately equal to a 0.5 McFarland density standard) in 0.5 ml of 0.85% saline.
- On a glass slide or reaction card, add 10 µl (1 droplet) of the latex reagent and 10 µl of the cell suspension. Mix the two suspensions together.
- After 10-30 seconds, observe the latex agglutination reaction at an angle with oblique lighting.
Reading the latex agglutination results
- A positive reaction is indicated by agglutination (cells clumping together) appearing within 5-10 seconds.
- A negative reaction is indicated by no agglutination appearing within 5-10 seconds.
Troubleshooting
- The latex agglutination reaction should be examined within 5-10 seconds. If the reaction time exceeds 30 seconds, false positive reactions may occur.
Quality control
- Each lot of latex suspension should be tested for positive agglutination reactions using S. pneumoniae reference strains with known capsular serotypes.
-
Quellung reaction
For proper quellung-based serotyping, a high quality microscope is required. A positive quellung or Neufeld reaction is the result of the binding of the capsular polysaccharide of pneumococci with type specific antibody contained in the typing antiserum. Pneumococcal typing sera are commercially available as pooled, group, or serotype-specific (see http://www.ssi.dk/English.aspx). It is recommended to initially test with pooled antisera in succession until a positive reaction is observed. Typing should then proceed by testing with individual group and serotype-specific antisera included in the antisera pool that gave a positive reaction to determine the serogroup and serotype. An antigen-antibody reaction causes a change in the refractive index of the capsule so that it appears “swollen” and more visible. After the addition of a counter stain (methylene blue), the pneumococcal cells stain dark blue and are surrounded by a sharply demarcated halo which represents the outer edge of the capsule. The light transmitted through the capsule appears brighter than either the pneumococcal cell or the background. Single cells, pairs, chains, and even clumps of cells may have positive quellung reactions.
Performing the quellung reaction
- Grow the isolate(s) to be tested for 18-24 hours on a BAP at 35-37°C with ~5% CO2 (or in a candle-jar).
- From overnight growth on the BAP, use a sterile loop to prepare a light to moderate cell suspension (approximately equal to a 0.5 McFarland density standard) in 0.5 ml of 0.85% saline.
- Optimum quellung reactions can be observed when there are 25-50 cells visible in a microscopic field at 1000X magnification.
- Dispense equal amounts of antiserum (5 µl) and methylene blue (5 µl) onto a microscope slide. Add approximately 0.2-1.0 µl of the diluted cell suspension and mix all three with a pipette tip.
- Cover the suspension with a 22 mm2 square cover-slip and incubate at room temperature (25°C) for 10-15 minutes.
- Do not allow the fluid on the slide to dry.
- Examine the slide at 1000X using an oil immersion lens.
- Begin testing with pooled antisera. Once a positive reaction is obtained, proceed with individual group and serotype-specific antisera included in the pooled antisera that gave the positive reaction to determine the serogroup and serotype.
Reading the quellung reaction results
- A positive quellung reaction is observed when the capsule appears as a sharply demarcated halo around the dark blue stained cell (Figure 7).
- A negative quelling reaction is observed when there is no appearance of a clear, enlarged halo surrounding the stained cell.
Troubleshooting
- In some instances, observing a positive reaction can be difficult. Prepare and read all quellung reactions on the same day that the cell suspension is prepared.
- When reading the reactions, look for free floating single or paired cells.
- Agglutination (cells clumping together) is NOT a positive quellung reaction.
- If a quellung reaction is not observed in any of the antisera pools, the strain may be non-typeable, but identification of the strain as S. pneumoniae should be confirmed by optochin susceptibility and bile solubility testing.
Quality control
- Each lot of antisera received should be tested for positive quellung reactions using S. pneumoniae reference strains with known capsular serotypes.
-
Determining S. pneumoniae capsular serotypes using PCR-based methods
The high cost of antisera, subjectivity in interpretation, and technical expertise requirements associated with these serologic methods have resulted in the development of PCR-based serotyping systems. PCR-based serotyping has the potential to overcome some of the difficulties associated with serologic testing and assays for direct detection of serotypes from clinical specimens are a valuable aid in surveillance, particularly in situations where culture is insensitive.
Conventional PCR-based methods have been developed to determine the serotypes of S. pneumoniae specimens. Conventional multiplex PCR assays are available for detecting 40 of the 93 S. pneumoniae serotypes. Schemes for testing based on the strains that are historically and/or currently circulating in specific regions are listed in Chapter 10: PCR Methods. Published real-time PCR serotyping assays are also available for many common pneumococcal serotypes.
-
General methods for genotyping S. pneumoniae
The continued study of the "seroepidemiology" of pneumococcal disease and carriage isolates is important for understanding selective effects upon regional population structures of this species. Trends in pneumococcal carriage and disease epidemiology are influenced by selective factors in the environment, such as the use of antimicrobial drugs and the introduction of conjugate vaccines. Understanding questions related to long-term effects of such pressures on the pneumococcal population require precise isolate characterization using molecular methods to characterize the strains at the genetic level. Chapter 12: Molecular Methods describes some of the most common typing methodologies used to differentiate S. pneumoniae and includes pulsed-field gel electrophoresis (PFGE), multilocus sequence typing (MLST) and the use of more variable loci such as the penicillin binding protein (pbp) genes and the pneumococcal surface protein (pspA) gene.
- Page last reviewed: April 15, 2016
- Page last updated: March 15, 2012
- Content source:


 ShareCompartir
ShareCompartir