Flu Vaccination Coverage, United States, 2014-15 Influenza Season
Data sources: National Immunization Survey-Flu (NIS-Flu) and Behavioral Risk Factor Surveillance System (BRFSS)
Data Sources and Methods | Limitations
Influenza (flu) can cause serious illness and death, particularly in younger and older persons, pregnant women, and those with certain medical conditions. To reduce sickness and death caused by influenza in the United States, the Advisory Committee on Immunization Practices recommends annual flu vaccination for all persons aged ≥6 months who do not have contraindications.(1) Optimally, people should receive their flu vaccinations before the start of flu activity in the community; therefore if possible, health care providers should offer flu vaccinations by October, and continue to offer vaccinations as long as flu viruses are circulating.(1)
For this report, CDC analyzed data from the National Immunization Survey-Flu (NIS-Flu) for children 6 months through 17 years and the Behavioral Risk Factor Surveillance System (BRFSS) for adults ≥18 years to estimate national flu vaccination coverage from the 2014–15 flu season. Coverage estimates are presented by age group, race/ethnicity, and month of vaccination with additional information for adults with certain medical conditions (e.g., asthma, diabetes, heart disease, chronic obstructive pulmonary disease, or cancers other than skin cancer) that put them at higher risk for flu-related complications.
Additional estimates of flu vaccination coverage by age and racial/ethnic groups for the 2014–15 and earlier seasons for each state, each Health and Human Services (HHS) region, and the United States are provided in FluVaxView as interactive maps, figures, and tables. Selected estimates for local areas and territories are also available (2014-15 Estimates for Local Areas and Territories ). Coverage estimates for pregnant women and healthcare personnel are reported in the MMWR.
Key Findings
- Flu vaccination coverage among children for the 2014-15 season did not change from the 2013-14 season.
- Flu vaccination coverage among adults increased by 1.4 percentage points for the 2014-15 season compared to the 2013-14 season.
- State variability in child and adult flu vaccination coverage continues to be large. (Interactive Report)
Who Was Vaccinated?
Coverage by Age Group
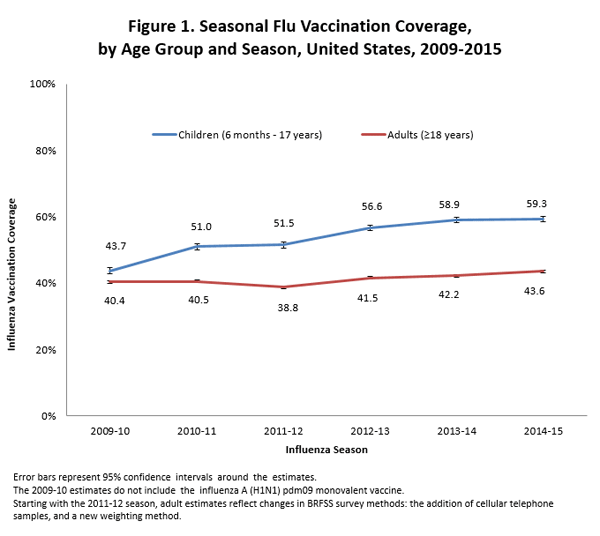
- Among all people ≥6 months, flu vaccination coverage during the 2014–15 flu season was 47.1%, which was a 0.9 percentage point increase compared to the 2013-14 season (46.2%).
- State-specific flu vaccination coverage among all people ≥6 months ranged from 39.2% (Florida) to 59.6% (South Dakota). (Interactive Report)
Children (6 months through 17 years)
- Flu vaccination coverage among children 6 months through 17 years decreased with increasing age:
- 6–23 months: 74.6%
- 2–4 years: 67.8%
- 5–12 years: 61.8%
- 13–17 years: 46.6%
- Among children 6 months through 17 years, coverage with one or more doses of flu vaccine was 59.3%, not statistically different from the 2013–14 season (58.9%).
- State-specific flu vaccination coverage for children 6 months through 17 years ranged from 45.3% (Montana) to 78.6% (Rhode Island). (Interactive Report)
| Age Group |
Unweighted Sample Size |
%‡± 95% CI§ |
Difference from the 2013-14 Season ± 95% CI |
|---|---|---|---|
| 6 months−17 years |
128,143 |
59.3 ± 0.8 |
0.4 ± 1.1 |
| 6 months−4 years |
37,626 |
70.4 ± 1.3 |
0.0 ± 1.8 |
| 6−23 months |
14,126 |
74.6 ± 1.9 |
0.3 ± 2.8 |
| 2−4 years |
23,500 |
67.8 ± 1.7 |
-0.3 ± 2.5 |
| 5−17 years |
90,517 |
55.8 ± 0.9 |
0.5 ± 1.3 |
| 5−12 years |
59,075 |
61.8 ± 1.1 |
0.8 ± 1.6 |
| 13−17 years |
31,442 |
46.6 ± 1.4 |
0.2 ± 2.1 |
For estimates for children by place of vaccination, please see Place of Flu Vaccination 2014-15.
- Coverage among adults 18 years and older increased with increasing age:
- 18–49 years: 33.5%
- 50–64 years: 47.0%
- ≥65 years: 66.7%
- Among adults ≥18 years, coverage was 43.6%, which was 1.4 percentage points higher than coverage in the 2013–14 season (42.2%).
- State-specific coverage for adults 18 years and older ranged from 36.3% (Nevada) to 58.1% (South Dakota). (Interactive Report)
Coverage by Sex
Children (6 months through 17 years)
- There were no differences in flu vaccination coverage between male and female children.
- For adults, flu vaccination coverage was higher among females than males for every age group except adults ≥65 years and adults with high risk conditions aged 18-64 years and 18-49 years.
Coverage by Race/Ethnicity
- Among people ≥6 months, coverage for non-Hispanic whites (48.5%) was higher than that of non-Hispanic blacks (43.8%), Hispanics (44.3%), and people of other or multiple races (44.3%).
- In addition to the previously mentioned differences, coverage for Asians (51.0%) was higher than non-Hispanic blacks (43.8%), Hispanics (44.3%), AI/ANs (45.2%), and people of other or multiple races (44.3%). For all other racial/ethnic group comparisons there were no statistically significant differences.
- Among all people ≥6 months, coverage during the 2014–15 season increased by 1.1 percentage points for non-Hispanic whites and 2.3 percentage points for non-Hispanic blacks compared to the 2013–14 season; there were no increases in the other racial/ethnic groups.
Children (6 months through 17 years)
- Non-Hispanic white children (56.0%) had lower flu vaccination coverage than Hispanic children (64.2%), Asian children (72.1%), AI/AN children (67.0%), and children of other or multiple races (60.0%). White children had similar coverage to non-Hispanic black children (58.3%).
- Additionally, non-Hispanic black children (58.3%) had lower flu vaccination coverage than Hispanic children (64.2%), Asian children (72.1%), and AI/AN children (67.0%); Asian children (72.1%) had higher coverage than Hispanic children (64.2%) and children of other or multiple races (60.0%), and Hispanic children (64.2%) and AI/AN children (67.0%) had higher coverage than children of other or multiple races (60.0%). For all other racial/ethnic group comparisons, there were no statistically significant differences.
- Among children, coverage during the 2014–15 season did not change compared to the 2013–14 season for any of the racial/ethnic groups.
For additional race/ethnicity estimates by age group, please see attached table: Table 6 Supplement [16 KB]
- Among adults, coverage for non-Hispanic whites (46.7%) was higher than coverage for non-Hispanic blacks (38.7%), Hispanics (35.0%), AI/ANs (40.7%), and adults of other or multiple races (37.4%); non-Hispanic whites had similar coverage to Asians (44.4%).
- Additionally among adults, non-Hispanic blacks (38.7%) and AI/ANs (40.7%) had higher coverage than Hispanics (35.0%), while Asians (44.4%) had higher coverage than non-Hispanic blacks (38.7%), Hispanics (35.0%), and adults of other or multiple races (37.4%). For all other racial/ethnic group comparisons, there were no statistically significant differences.
- Among adults, coverage during the 2014–15 season increased by 1.3 percentage points for non-Hispanic white adults and 3.1 percentage points for non-Hispanic black adults compared to the 2013–14 season; there were no increases in the other racial/ethnic groups
For additional race/ethnicity estimates by age group, please see attached table: Table 7 Supplement [16 KB]
Coverage by Month
Children (6 months through 17 years)
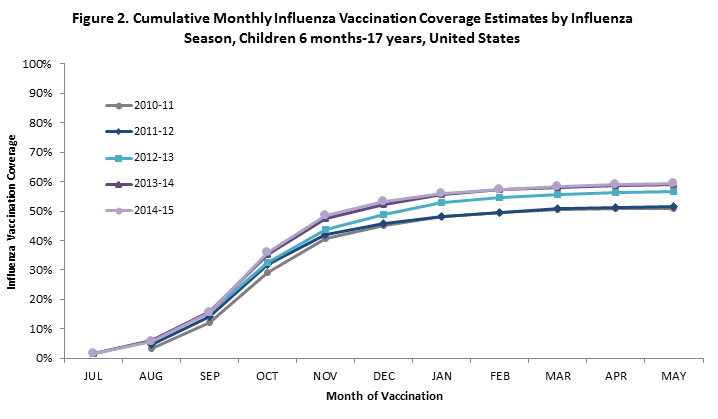
- Among children 6 months through 17 years, cumulative coverage across the months of vaccination was similar for the 2014–15 season compared to the 2013–14 season.
Data Sources and Methods | Limitations
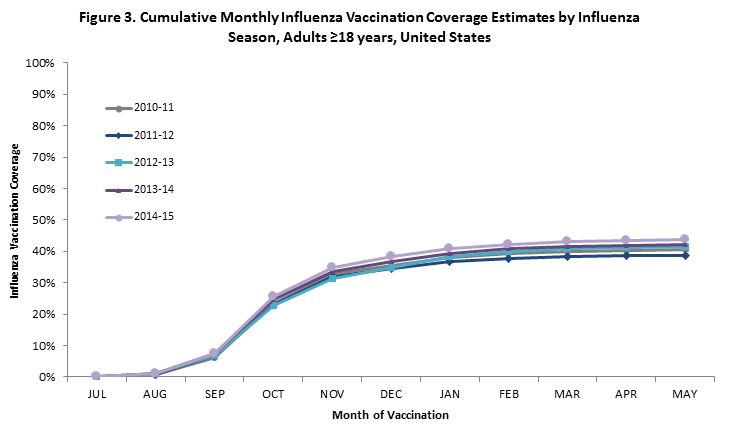
- Among adults ≥18 years, cumulative coverage by the end of May was slightly higher for the 2014–15 season compared to the 2013–14 season (Figure 3).
Data Sources and Methods | Limitations
Learn more about the percentage of vaccinations given each month among vaccinated children.
Estimated Number of Persons Vaccinated
Based on reports of vaccination from survey respondents, the estimated number of persons with reported receipt of one or more seasonal flu vaccinations was 42.1 million (95% Confidence Interval [CI] 41.5–42.7 million) children (6 months through 17 years) and 106.0 million (95% CI 105.0–107.0 million) adults (≥18 years), for an estimated 148.1 million (95% CI 146.6–149.7 million) people vaccinated against seasonal flu during July 2014 through May 2015 among the civilian, non-institutionalized U.S. population. These estimates do not include military or institutionalized persons who were vaccinated and do not include second doses given to children. However, the actual number of doses of flu vaccine distributed during the 2014-15 season was 147.8 million, indicating that the flu vaccination coverage estimates reported in this article are high. Over-estimates of doses may be due to a combination of factors including respondents having higher coverage than persons not surveyed (response bias), recall bias, or other factors. Examples of studies in which medical record validation has been compared to adult patient or parent report of vaccination estimated that coverage by parental report was seven percentage points too high for children 6-59 months, and coverage by self-report was 5-11 percentage points too high for adults ≥65 years.(2;3) Thus, while numbers of doses administered cannot be validated with this data, the surveys do provide important information for following trends in vaccination over time among different populations.
What Can Be Done?
For children, flu vaccination coverage was similar for the 2014-15 season compared to the 2013-14 season while there was a small increase for adults. Differences in coverage among racial/ethnic populations by age group and variation in coverage by state were noted and consistent with findings from prior flu seasons. Flu vaccination coverage for the 2014-15 season for all groups, except children 6-23 months, was below the Healthy People 2020 targets§§ of 70% vaccination coverage for persons 6 months through 17 years and ≥18 years.(4)
The effectiveness of the flu vaccine is in part based on the match between circulating flu strains and those included in the vaccine. Vaccination can reduce the risk of influenza and its complications even during a season when the vaccine match for one flu virus strain is not optimal, as in 2014-15, since the vaccine contains protection against 3-4 influenza virus strains. In most flu seasons, more than one flu virus type circulates. For these reasons, even during seasons when there is a less than ideal match, CDC continues to recommend flu vaccination for everyone 6 months and older.
Efforts are needed to continue to increase flu vaccination coverage in the United States, including:
- Encouraging use of evidence-based practices at medical sites to increase access to vaccination services (e.g., reducing client costs and vaccination programs in schools and WIC settings), increasing community demand for vaccinations (e.g., client reminder/recall systems), and ensuring that all those who visit a provider during the flu season receive a vaccination recommendation and offer from their provider (e.g., standing orders, and provider reminders).(5)
- Expanding access through use of non-traditional settings for vaccination (e.g., pharmacy, workplace, and school venues) to reach individuals who may not visit a traditional physician’s office during the flu season.(6)
- Broadening use of interventions to remove barriers to accessing vaccination.(7)
- Encouraging multi-sector collaborations, including culturally relevant communications to reach specific target populations and implementing effective interventions to reduce vaccination disparities in the United States.(8)
- Additional strategies are described in the Community Guide for Preventive Services.(5)
Updated recommendations have been published for the 2015-16 flu season.(1) Updated information includes 1) the composition of U.S. seasonal flu vaccines; 2) the expected flu vaccine products available for the 2015-16 season; 3) an updated algorithm for determining the appropriate number of doses for children 6 months through 8 years of age; and 4) recommendations for the use of live attenuated influenza vaccine, inactivated and recombinant influenza vaccine formulations.(1)
Data Sources and Methods
CDC analyzed NIS-Flu and BRFSS data collected September (BRFSS) or October (NIS-Flu) 2014 through June 2015 (or as available) from all 50 states and the District of Columbia to estimate national and state level flu vaccination coverage for vaccines administered from July 2014 through May 2015 for the 2014–15 flu season. These findings were compared to 2013–14 flu season estimates. Estimates are also included as a supplemental table to this report for Guam, Puerto Rico, the Virgin Islands, and select local areas.
The NIS-Flu has three components: the NIS which includes households with children 19–35 months, the NIS-Teen which includes households with children 13–17 years, and a short flu vaccination module which is conducted for households with children 6–18 months and 3–12 years. The NIS-Flu is a national dual landline cellular list-assisted random-digit-dialed telephone survey of households. Respondents ≥18 years were asked if their child had received a flu vaccination since July 1, 2014 and, if so, in which month and year. The range of the Council of American Survey and Research Organizations (CASRO) response rates for the NIS-Flu across the components of the NIS-Flu were 55.9% to 64.8% for landline and 34.3% to 38.8% for cellular telephones.
BRFSS is an ongoing state-based monthly telephone survey which collects information on health conditions and risk behaviors from randomly selected persons ≥18 years among the non-institutionalized, U.S. civilian population. BRFSS respondents were asked if they had received a flu vaccine in the past 12 months, and if so, in which month and year. The median state BRFSS response rate was 47.5% for September–December 2014 and 46.2% for January–June 2015. Starting in 2011, BRFSS methods changed by adding persons in households with only cellular telephone service and improvements to weighting procedures; these changes were reflected in the 2011–12 and subsequent flu vaccination coverage estimates.(9)
Flu vaccination coverage estimates from both surveys were calculated using Kaplan-Meier survival analysis to determine the cumulative flu vaccination coverage (≥1 dose) July 2014 through May 2015 using monthly interview data collected September (BRFSS) or October (NIS-Flu) 2014 through June 2015. NIS-Flu data were used to estimate coverage for children 6 months through 17 years and BRFSS data were used to estimate coverage for adults ≥18 years. Coverage estimates for all persons ≥6 months were determined using combined state-level monthly estimates weighted by the age-specific populations of each state. (10) For 18.9% of vaccinated NIS-Flu participants and 6.3% of vaccinated BRFSS participants, month of vaccination was not reported and was imputed from donor pools matched for week of interview, age group, state of residence, and race/ethnicity. Information on high-risk conditions was missing for 1.1% of adults and race/ethnicity was missing for 1.4% of adults; adults with missing data are not included in the estimates by risk condition or race/ethnicity. Results from both surveys were weighted and analyzed with SAS and SUDAAN statistical software to account for the complex survey design. Differences between groups and between 2013–14 and 2014–15 seasons were determined using t-tests with significance at p<0.05. Differences mentioned in this report were statistically significant.
Limitations
The estimates in this report are subject to the following limitations. First, flu vaccination status was based on self or parental report and not validated with medical records and, thus, is subject to respondent recall bias.(2;3) NIS-Flu and BRFSS, as well as the National Health Interview Survey (NHIS), are all subject to respondent recall bias. Second, response rates for NIS-Flu and BRFSS surveys were low and nonresponse bias may remain even after weighting adjustments. A comparison of NIS-Flu estimates with those from NHIS suggests that the NIS-Flu estimates have a slight upward nonresponse bias.( 11;12) Third, combining NIS-Flu and BRFSS estimates allowed estimation of coverage for all persons ≥6 months; however, differences in survey methodology (e.g., different sampling frame, survey design, exact survey question wording, response rates and weighting) may result in different levels of bias that are averaged for this group. Fourth, the number of persons vaccinated was overestimated, evidenced by a higher number vaccinated than doses distributed as has occurred previously.(13) Finally, some age-by-state-specific estimates in the accompanying interactive reports may not be reliable due to large confidence intervals. Estimates flagged as potentially unreliable should be interpreted with caution.
Authors
Tammy A. Santibanez, PhD; Katherine E. Kahn, MPH; Yusheng Zhai, MSPH; Alissa O’Halloran, MSPH; Nick Davis, MS; Carolyn B. Bridges, MD; Peng-Jun Lu, MD, PhD; Stacie M. Greby, DVM, MPH; Walter W. Williams, MD, MPH; James A. Singleton, PhD
Related Links
National Immunization Survey-Flu (NIS-Flu):
Behavioral Risk Factor Surveillance System (BRFSS):
NIS-Flu/BRFSS vaccination coverage reports:
General information about flu:
Footnotes
* Estimates of the percentage of people vaccinated are based on interviews conducted beginning September (BRFSS) or October (NIS-Flu) 2014 through June 2015 and reported vaccinations from July 2014 through May 2015.
† Excludes U.S territories.
‡ Percentage vaccinated. Percentages are weighted to the non-institutionalized U.S. civilian population. Month of vaccination was imputed for respondents with missing month of vaccination data.
§ Confidence interval (CI) half-widths.
|| Statistically significant difference between the 2014-15 season and the 2013-14 season by t-test (P<0.05).
¶ Selected high-risk conditions; includes people with asthma, diabetes, heart disease, chronic obstructive pulmonary disease, or cancers other than skin cancer.
** Statistically significant difference between male and female estimates by t-test (P<0.05).
†† Race is reported by respondent; people of Hispanic ethnicity may be of any race.
‡‡ Includes Native Hawaiian or other Pacific Islander, multiracial, and other races.
§§ The National Health Interview Survey (NHIS) is the data source used to monitor the Healthy People objectives for influenza vaccination (IID-12.11-14). Final NHIS estimates for the 2014-15 season will be available by September 2016. Information about these objectives is available online at: Immunization and Infectious Diseases. A comparison of estimates from NIS-Flu and BRFSS to NHIS is available online at 2013-14 NHIS, BRFSS, and NIS-Flu Influenza Data.
References
(1) Grohskopf LA, Sokolow LZ, Olsen SJ, Bresee JS, Broder KR, Karron RA. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2015-16 influenza season. MMWR 2015;64:818-825.
(2) MacDonald R, Baken L, Nelson A, Nichol KL. Validation of self-report of influenza and pneumococcal vaccination status in elderly outpatients. Am J Prev Med 1999;16:173-177.
(3) Brown C, Clayton-Boswell H, Chaves SS et al. Validity of parental report of influenza vaccination in young children seeking medical care. Vaccine 2011;29:9488-9492.
(4) U.S.Department of Health and Human Services. Healthy People 2020. Topics & Objectives-Immunization and Infectious Diseases. http://www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives [serial online] 2015; Accessed September 11, 2015.
(5) Guide to Community Preventive Services. Increasing appropriate vaccination. www thecommunityguide org/vaccines/index html [serial online] 2013; Accessed July 30, 2013.
(6) Murphy PA, Frazee SGCJP, Cohen E, Rosan JR, Harshburgher DE. Pharmacy provision of influenza vaccinations in medically underserved communities. J Am Pharm Assoc 2012;52:70.
(7) Poland GA, Shefer AM, McCauley M, Webster PS, Whitley-Williams PN, Peter G. Standards for adult immunization practices. Am J Prev Med 2003;25:144-150.
(8) CDC. CDC health disparities and inequalities report–United States, 2013. MMWR 2013;62:3-5.
(9) CDC. Methodologic changes in the Behavioral Risk Factor Surveillance System in 2011 and potential effects on prevalence estimates. MMWR 2012;61:410-413.
(10) Furlow-Parmley C, Singleton JA, Bardenheier B, Bryan L. Combining estimates from two surveys: an example from monitoring 2009 influenza A(H1N1) pandemic vaccination. Stat Med 2012;31:3285-3294.
(11) Santibanez TA, Lu PJ, O’Halloran A, Meghani A, Grabowsky M, Singleton JA. Trends in Childhood Influenza Vaccination Coverage–U.S., 2004-2012. Public Health Rep 2014;129:417-427.
(12) CDC. Surveillance of influenza vaccination coverage–United States, 2007-08 through 2011-12 influenza seasons. MMWR CDC Surveill Summ 2013;62:1-28.
(13) CDC. Interim results: state-specific seasonal influenza vaccination coverage–United States, August 2009-January 2010. MMWR Morb Mortal Wkly Rep 2010;59:477-484.
- Page last reviewed: June 23, 2016
- Page last updated: June 23, 2016
- Content source:
- Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases (NCIRD)
- Page maintained by: Office of the Associate Director for Communication, Digital Media Branch, Division of Public Affairs


 ShareCompartir
ShareCompartir