Medical Office Telephone Evaluation of Patients with Possible Influenza
The flowchart below is designed to be used when influenza is circulating in the community. This tool may help medical office staff triage calls from patients with flu-like symptoms and identify when it might be appropriate to initiate antiviral treatment before an office visit. Patient triage or prescribing of prescription medicines should be done under the direction of a licensed physician or other licensed health care provider.
Also available as PDF [357 KB, 1 page]
** High-risk patients include:
- Children younger than 2 years (although all children younger than 5 years are considered at higher risk for complications from influenza, the highest risk is for those younger than 2 years);
- Adults aged 65 years and older;
- Persons with chronic pulmonary (including asthma), cardiovascular (except hypertension alone), renal, hepatic, hematological (including sickle cell disease), and metabolic disorders (including diabetes mellitus), or neurologic and neurodevelopment conditions (including disorders of the brain, spinal cord, peripheral nerve, and muscle such as cerebral palsy, epilepsy [seizure disorders], stroke, intellectual disability [mental retardation], moderate to severe developmental delay, muscular dystrophy, or spinal cord injury);
- Persons with immunosuppression, including that caused by medications or by HIV infection;
- Women who are pregnant or postpartum (within 2 weeks after delivery);
- Persons aged younger than 19 years who are receiving long-term aspirin therapy;
- American Indians/Alaska Natives;
- Persons who are extremely obese (i.e., body-mass index is equal to or greater than 40); and
- Residents of nursing homes and other chronic-care facilities.
For more information, see Antiviral Drugs – Recommendations of the ACIP: Information for Health Care Professionals.
Flowchart Descriptive Text
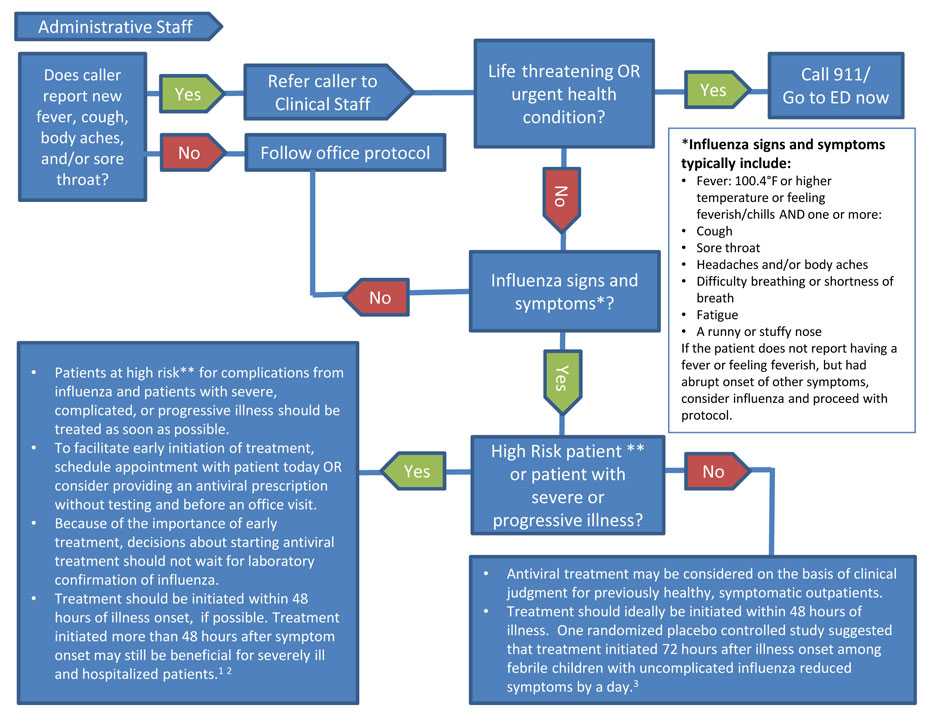
For Administrative Staff ǂ: Does the caller report new fever, cough, body aches, and/or sore throat? If Yes, refer the caller to Clinical Staff. If No, follow office protocol.
For Clinical Staff: Is there a life-threatening OR urgent health condition? If Yes, call 911/Go to an emergency department (ED) now.
If No, does caller have influenza signs and symptoms*? If No, follow office protocol.
If Yes, is the caller a high-risk patient** or patient with severe or progressive illness?
If No to high-risk patient:
- Antiviral treatment may be considered on the basis of clinical judgment for previously healthy, symptomatic outpatients.
- Treatment should ideally be initiated within 48 hours of illness. One randomized placebo controlled study suggested that treatment initiated 72 hours after illness onset among febrile children with uncomplicated influenza reduced symptoms by a day.3
If Yes to high-risk patient:
- Patients at high risk** for complications from influenza and patients with severe, complicated, or progressive illness should be treated with antivirals as soon as possible.
- To facilitate early initiation of treatment, schedule appointment with patient today OR consider providing an antiviral prescription without testing and before an office visit.
- Because of the importance of early treatment, decisions about starting antiviral treatment should not wait for laboratory confirmation of influenza.
- Treatment should be initiated within 48 hours of illness onset, if possible. Treatment initiated more than 48 hours after symptom onset may still be beneficial for severely ill and hospitalized patients.1-2
* Influenza signs and symptoms typically include:
- Fever: A 100.4°F or higher temperature or feeling feverish/chills AND one or more:
- Cough
- Sore throat
- Headaches and/or body aches
- Difficulty breathing or shortness of breath
- Fatigue
- A runny or stuffy nose
If the patient does not report having a fever or feeling feverish, but had abrupt onset of other symptoms, consider influenza and proceed with protocol.
References
- Louie JK, Yang S, Acosta M, et al. Treatment with neuraminidase inhibitors for critically ill patients with influenza A (H1N1)pdm09. Clin Infect Dis. 2012; 55(9): 1198-204.
- Yu H, Feng Z, Uyeki TM, et al. Risk factors for severe illness with 2009 pandemic influenza A (H1N1) virus infection in China. Clin Infect Dis. 2011; 52(4): 457-65.
- Fry AM, Goswami D, Nahar K, et al. Efficacy of oseltamivir treatment started within 5 days of symptom onset to reduce influenza illness duration and virus shedding in an urban setting in Bangladesh: a randomised placebo-controlled trial. Lancet Infect Dis. 2014; 14(2): 109-18.
- Page last reviewed: February 3, 2017
- Page last updated: February 3, 2017
- Content source:
- Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases (NCIRD)
- Page maintained by: Office of the Associate Director for Communication, Digital Media Branch, Division of Public Affairs


 ShareCompartir
ShareCompartir