Full and Partial Flu Vaccination Coverage in Young Children, Six Immunization Information System Sentinel Sites
On This Page
DATA SOURCE: Immunization Information Systems (IIS) data from IIS Sentinel Sites.
Figure 1.
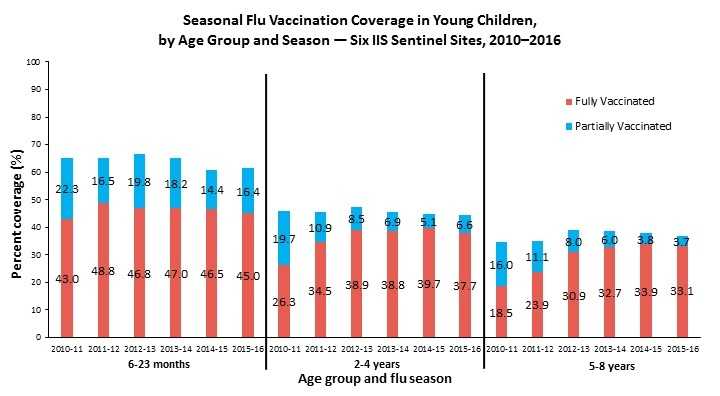
Footnote: Census data were used as denominators, and IIS data from the six IIS Sentinel Sites were used as numerators. Full vaccination coverage was classified as having received at least the recommended number of flu vaccine doses (one or two), based on ACIP recommendations for that season. Partial vaccination coverage was classified as having received only one flu vaccine dose when recommended to receive two doses.
Influenza (flu) viruses can cause mild to severe illness, and severe illnesses may result in hospitalization or death. The Advisory Committee on Immunization Practices (ACIP) recommends all children 6 months to 18 years receive annual flu vaccination, and children 6 months through 8 years receive two doses in their first vaccination season to achieve adequate immune response (1). Children who previously received flu vaccines may not require two doses in the current season; however, in some circumstances, two doses are still necessary, e.g., when a new strain is added to a flu vaccine. ACIP updates its flu vaccination recommendation each year and may recommend two doses to children even after their first flu seasons depending on their vaccination histories (2–7).
For this report, CDC analyzed data for >2.8 million children from six IIS Sentinel Sites for the 2010–11 through 2015–16 flu seasons, which were approximately 10% of the U.S. pediatric population. Full and partial flu vaccination coverage among children 6 months through 8 years is presented by age group and flu season.
Additional information on flu vaccination coverage for the United States are provided at FluVaxView as interactive maps, figures, and tables.
Key Findings
- From the 2010-11 through the 2015-16 flu season, full flu vaccination coverage was generally unchanged (≤2 percentage point change) in the 6–23 month olds, and increased (>10 percentage point change) in the 2–4-year and 5–8-year groups.
- From the 2014-15 to the 2015-16 flu season, full flu vaccination coverage remained generally unchanged (≤2 percentage point change) in all three age groups.
- From the 2014-15 to the 2015-16 flu season, the flu vaccination coverage of ≥1 dose remained generally unchanged (≤2 percentage point change) in all three age groups.
In addition to efforts to increase flu vaccination coverage in general, the following efforts may improve two-dose compliance and ultimately improve full vaccination coverage:
- Improved messaging of the two-dose flu vaccine recommendations is needed for providers and parents.
- Providers are encouraged to submit data to IIS to enhance the system, and use consolidated vaccination histories in IIS to assess children’s eligibility for two doses, especially for older children, who may be perceived to not need two doses.
Data Source & Methods
This is an update of data presented previously, and data source and methods have been described in reference (8) (https://www.ncbi.nlm.nih.gov/pubmed/27670074).
Limitations
This analysis is subject to limitations. First, data from six IIS Sentinel Sites might not represent the U.S. population. Second, incomplete vaccination histories in IIS data may underestimate vaccination coverage and two-dose compliance, especially in older children who visit their physician’s office less frequently but receive flu vaccination in other places. However, child participation rate was >95% for each IIS Sentinel Site (9), which reduces potential underestimations for flu vaccination rates.
Authors (and affiliation)
Xia Lin, PhD, MSPH; Loren Rodgers, PhD
Immunization Services Division, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention.
Related Links
General information about IIS and IIS Sentinel Sites:
General information about flu:
References/Resources
1.Centers for Disease Control and Prevention. Prevention and Control of Influenza: Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports / Centers for Disease Control. 2008 Aug 8;57(RR07):1-60.
2.Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports / Centers for Disease Control. 2010 Aug 6;59(RR-8):1-62.
3.Centers for Disease Control and Prevention. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morbidity and mortality weekly report. 2011 Aug 26;60(33):1128-32.
4.Centers for Disease Control and Prevention. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)–United States, 2012-13 influenza season. MMWR Morbidity and mortality weekly report. 2012 Aug 17;61(32):613-8.
5.Centers for Disease Control and Prevention. Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices–United States, 2013-2014. MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports / Centers for Disease Control. 2013 Sep 20;62(RR-07):1-43.
6.Grohskopf LA, Olsen SJ, Sokolow LZ, Bresee JS, Cox NJ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) — United States, 2014-15 influenza season. MMWR Morbidity and mortality weekly report. 2014 Aug 15;63(32):691-7.
7.Grohskopf LA, Sokolow LZ, Olsen SJ, Bresee JS, Broder KR, Karron RA. Prevention and Control of Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2015-16 Influenza Season. MMWR Morbidity and mortality weekly report. 2015 Aug 7;64(30):818-25.
8.Lin X, Fiebelkorn AP, Pabst LJ. Trends in compliance with two-dose influenza vaccine recommendations in children aged 6 months through 8 years, 2010-2015. Vaccine. 2016 Sep 23.
9.Centers for Disease Control and Prevention. Immunization Information Systems Annual Report (IISAR) – IISAR participation rates and maps (2015), https://www.cdc.gov/vaccines/programs/iis/annual-report-iisar/rates-maps-table.html, accessed December 2016.
- Page last reviewed: January 30, 2017
- Page last updated: January 30, 2017
- Content source:
- Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases (NCIRD)
- Page maintained by: Office of the Associate Director for Communication, Digital Media Branch, Division of Public Affairs


 ShareCompartir
ShareCompartir