Multistate Outbreak of Fungal Meningitis and Other Infections –Resources for Clinicians
On October 30, 2015, CDC updated its web resources for patients and clinicians. Patients affected by tainted steroid injections from the New England Compounding Center continue to receive treatment for their infections and clinicians should continue to monitor patient recovery. All relevant materials for patients and clinicians concerning the multistate outbreak of fungal meningitis and other infections are located on this page.
Update - October 30, 2015
Background
The following update provides information on clinical follow-up of patients affected in the unprecedented multistate invasive fungal infection outbreak associated with contaminated methylprednisolone injections in late 2012 and 2013. Many of the findings and recommendations come from a core group of public health, clinical providers, and subject matter experts. In February 2014, the group met to discuss current and ongoing clinical challenges, review patient and clinical guidance, and finalize plans for the long-term follow-up of patients. This page expands on the October 30, 2015 MMWR Notes from the Field summarizing conclusions reached during that meeting and subsequent information about the current state of the outbreak.
For background on the outbreak please visit: https://www.cdc.gov/hai/outbreaks/meningitis.html
The final update to the outbreak website was on October 23, 2013. At that time, 751 cases meeting the CDC case definition for confirmed and probable cases had been identified. Two additional cases have been identified, for a total of 753 cases to date. The first of these additional cases occurred in 2013 but was only identified retrospectively during a long-term follow up study of about 500 patients being conducted by the Mycoses Study Group Education and Research Consortium. The second case became symptomatic in November 2014, developing meningitis. CDC is unaware of any additional confirmed, probable, or suspected cases related to this outbreak.
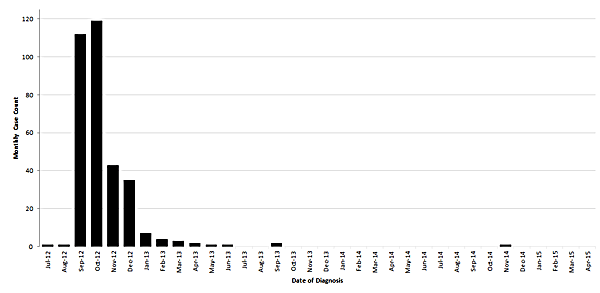
Monthly case counts of fungal infections associated with contaminated lots of methylprednisolone acetate based on week of diagnosis – United States, July 2012 to April 2015
Overall clinical picture
Most cases represented single site diagnoses (599/753; 80%), including 233 cases with meningitis, 326 with parameningeal infections, 33 with peripheral joint infections, and 7 with posterior circulation infarct presumed to be due to infection. The remaining cases had two concurrent site infections (154/753; 21%) including 152 cases with meningitis and parameningeal infection and two with parameningeal and peripheral joint infection. A detailed description of the original outbreak investigation and case patients is available here.1
Infections of the peripheral joints
Over 12% of exposures to contaminated steroid injections involved injection into peripheral joints. Currently, less than 5% of reported infections (35/753) involve the peripheral joints.1 The reason for this difference remains unclear.
Antifungal medication duration and infection relapse
Preliminary information provided by attending clinical providers indicates that patients in whom treatment was indicated received antifungal medication for approximately 6 months. A small subset of patients received long-term (>1 year), and possibly lifelong, antifungal therapy. Most patients who have completed treatment have done well with little evidence of recurrent or relapse infection. Overall, CDC is aware of 8 cases of infection relapse (reestablishment of active infection following treatment thought to have been successful) for an incidence of relapse of 1% (8/753). This relapse incidence is lower than the prevalence of relapse observed for other invasive fungal infections. A brief description of one confirmed relapse case has been published here.2 Among the six relapsed cases with known time from initial cessation of antifungal therapy to relapse date, the median time to relapse was 90 days (range 20 days–21 months). The relapse occurring 21 months post-cessation of therapy was recently identified and highlights the need for continued vigilance among persons involved in this outbreak.
The role of MRI
Clinical recommendations provided during the primary outbreak period included contrast-enhanced MRI (magnetic resonance imaging) for anyone who received a contaminated injection and experienced new or worsening symptoms at or near the injection site. Changes observable on MRI consistent with a fungal infection have proven valuable in the identification of cases, especially in patients with a history of significant baseline pain.3 Diagnostic MRI changes, however, have been noted to persist after treatment and resolution of symptoms. Continued MRI abnormalities without corresponding clinical findings (i.e., continued or worsening symptoms and/or lab tests indicating infection) should not be considered sufficient rationale to continue treatment or determine infection recurrence.
Persistent clinical features of infection
Although the majority of patients with follow-up data have reported full resolution of symptoms following successful treatment, residual symptoms have been observed in a minority of patients. The most notable sequelae have been worsening pain and the development of persistent mild to moderate cognitive impairment (e.g., impaired concentration, loss of memory) following treatment of central nervous system (CNS) infections. Treatment options for persistent pain and the identification of factors associated with these persistent cognitive changes remain an active area of inquiry.
(1-3)-β-D-glucan (BDG)
The fungal marker (1-3)-β-D-glucan (BDG) in cerebrospinal (CSF) may have value for the monitoring of clinical response to antifungal treatment. Serial CSF clinical specimens were tested to evaluate BDG level longitudinally. This assay may have additional value in determining and monitoring for infection recurrence.4 Measurement of BDG in CSF is a research test and is not approved by the Food and Drug Administration.
Surgical procedures following infection
There is a hypothetical possibility that fungal contamination once introduced into the body may remain present, but inactive, in exposed non-case patients and case-patients who have recovered from their initial infections. For this reason, there were concerns that a surgical procedure in the area that was previously exposed to contaminated steroid could then lead to a new active infection. Nevertheless, a substantial number of case-patients have undergone surgical procedures following steroid exposure with no reported complications attributable to their exposures.
Continuation of steroid injections following infection or exposure
It is not clear if or how additional epidural, paraspinal, or intra-articular steroid injections may increase the risk of fungal infection, or contribute to a recurrence of infection, in patients who received contaminated injections and are currently asymptomatic. Steroids are immunosuppressive and it is possible they could increase risk in patients with inactive, or sub-clinical, infection; however, the duration of this potential added risk resulting from prior exposure to a contaminated steroid product is still unknown.
CDC case definition
The CDC confirmed case definition required laboratory evidence of a fungal pathogen associated with this outbreak, and was developed as a public health surveillance tool to ensure standardized case finding. Therefore, CDC-confirmed cases should not be considered an exhaustive list of all individuals impacted. Throughout this outbreak, there have been many patients with negative results with CDC confirmatory testing who received antifungal treatment because they had concerning symptoms and other clinical features such as abnormal cerebrospinal fluid (CSF) or MRI findings.
CDC has always recommended that physicians should not rely on the CDC case definitions or results from any single laboratory test in deciding whether or not to treat a patient. Rather, they should treat if they believe the patient has an appropriate clinical picture and a history of steroid injection with one of the three contaminated lots of NECC methylprednisolone acetate.
Long-term follow-up of case patients
CDC and the Mycoses Study Group Education and Research Consortium are coordinating the collection of follow-up information describing the diagnostic and therapeutic approach and clinical course of case patients. Preliminary findings from this effort are expected in late 2015.
References
- 1. Smith RM, Schaefer MK, Kainer, MA, et al. Fungal infections associated with contaminated methylprednisolone injections. New Engl J Med. 2013 Oct; 369:1598-1609.
- 2. Smith RM, Tipple M, Chaudry MN, Schaefer MK, Park BJ. Relapse of Fungal Meningitis Associated with Contaminated Methylprednisolone. New Engl J Med2013 June; 368:2535-36.
- 3. Malani AN, Vandenberg DM, Singal B, et al. Magnetic resonance imaging screening to identify spinal and paraspinal infections associated with injections of contaminated methylprednisolone acetate. JAMA 2013 June; 309(23):2465-72.
- 4. Litvintseva AP, Lindsley MD, Gade L, et al. Utility of (1-3)-β-D-glucan testing for diagnostics and monitoring response to treatment during the multistate outbreak of fungal meningitis and other infections. Clin Infect Dis 2014 Mar; 58(5):622-30.
Case Definitions
Case Definitions for Fungal Meningitis and Other Infections
Probable Case:
A person who received a preservative-free methylprednisolone acetate (MPA) injection, with preservative-free MPA that definitely or likely came from one of the following three lots produced by the New England Compounding Center (NECC) [05212012@68, 06292012@26, 08102012@51], and subsequently developed any of the following:
- Meningitis[1] of unknown etiology following epidural or paraspinal injection2 after May 21, 2012;
- Posterior circulation stroke without a cardioembolic source and without documentation of a normal cerebrospinal fluid (CSF) profile, following epidural or paraspinal injection[2] after May 21, 2012;[3]
- Osteomyelitis, abscess or other infection (e.g., soft tissue infection) of unknown etiology, in the spinal or paraspinal structures at or near the site of injection following epidural or paraspinal injection [2] after May 21, 2012; or
- Osteomyelitis or worsening inflammatory arthritis of a peripheral joint (e.g., knee, shoulder, or ankle) of unknown etiology diagnosed following joint injection after May 21, 2012.
- Clinically diagnosed meningitis with one or more of the following symptoms: headache, fever, stiff neck, or photophobia, in addition to a CSF profile showing pleocytosis (>5 white blood cells, adjusting for presence of red blood cells by subtracting 1 white blood cell for every 500 red blood cells present) regardless of glucose or protein levels.
- Paraspinal injections include, but are not limited to, spinal facet joint injection, sacroiliac joint injection, or spinal or paraspinal nerve root/ganglion block.
- Patients in this category who do not have any documented CSF results should have a lumbar puncture performed if possible, using a different site than was used for the epidural injection when possible.
Confirmed Case:
A probable case with evidence (by culture, histopathology, or molecular assay) of a fungal pathogen associated with the clinical syndrome.
Frequently Asked Questions
Background
Beginning in September 2012, the Centers for Disease Control and Prevention (CDC), with state and local health departments and the Food and Drug Administration (FDA) began investigating a multistate outbreak of fungal infections among patients who received contaminated preservative-free methylprednisolone acetate (MPA) steroid injections from the New England Compounding Center (NECC). The infections identified as part of this investigation include fungal meningitis, a form of meningitis that is not contagious, localized spinal or paraspinal infections, and infections associated with injections in a peripheral joint space, such as a knee, shoulder, or ankle.
In the early stages of the outbreak, the majority of patients presented with meningitis. As the outbreak progressed, the majority of infections reported to CDC were patients with localized spinal or paraspinal infections (e.g., epidural abscess).
What Should Physicians Be Doing?
- Evaluate potentially exposed patients with new, ongoing, or persistent baseline symptoms.
- Report concerning cases to public health.
- Clinicians should report suspected cases to their state health department.
- Clinicians are also requested to report any suspected adverse events following use of these products to FDA’s MedWatch program at 1-800-332-1088 or www.fda.gov/medwatch.
- Use appropriate treatment for fungal meningitis, parameningeal infections, and joint infections.
Localized Spinal or Paraspinal Infection
There have been reports of localized spinal or paraspinal infections such as epidural abscesses and arachnoiditis among patients in this outbreak.
What are these conditions and their symptoms?
The majority of cases reported to CDC are localized spinal or paraspinal infections, including epidural abscess, phlegmon, arachnoiditis, discitis, or vertebral osteomyelitis, which were identified through Magnetic Resonance Imaging (MRI) with contrast. Some of these cases occurred in patients with persistent but baseline symptoms.
A spinal epidural abscess is characterized by inflammation and a collection of pus around the spine. Spinal epidural abscesses sometimes result in swelling in the affected area (e.g., near the site where contaminated steroid mediation was injected).
- Common symptoms can include fever, headache, back pain, and neurological problems (e.g., weakness, unusual changes in sensation)
Arachnoiditis is a disorder caused by the inflammation of the arachnoid, one of the membranes that surrounds and protects the nerves of the spinal cord. The condition can be caused by irritation from chemicals, infection, or direct injury to the spine.
- Symptoms can include numbness, tingling, and a characteristic stinging and burning pain in the lower back or legs. Some people with arachnoiditis may have debilitating muscle cramps, twitches, or spasms. The condition may also affect the bladder, bowel, and sexual function. In severe cases, arachnoiditis may cause paralysis of the lower limbs.
- For more information about arachnoiditis, see the National Institute of Neurological Disorders and Stroke’s website.
For more information on treatment guidance for these conditions, please see Interim Treatment Guidance for Central Nervous System and Parameningeal Infections Associated with Injection of Contaminated Steroid Products.
Who should be evaluated for a localized spinal or paraspinal infection?
As a part of continued monitoring of patients who received an injection with implicated MPA, clinicians should consider re-evaluating patients who received a spinal or paraspinal injection with implicated MPA for signs and symptoms suggestive of infection, including any symptoms at or near the site of their injection. Because of the prolonged incubation period for these infections, this guidance pertains both to patients who have not been previously evaluated and to those who have already had a prior negative evaluation (e.g., normal cerebrospinal fluid profile, normal findings on MRI) but continue to have complaints:
- In patients with new or worsening symptoms at or near the site of their injection, clinicians should obtain an MRI with contrast of the symptomatic area(s).
- In patients with persistent but baseline symptoms, clinicians should consider obtaining an MRI with contrast of the symptomatic area(s) because the presentation of spinal or paraspinal infections can be subtle, and may be difficult to distinguish from a patient’s baseline chronic pain.
- In some cases, radiologic evidence of abscess or phlegmon has become apparent on repeat MRI studies performed subsequent to an initially normal imaging procedure. Clinicians should therefore have a low threshold for repeat MRI studies in patients who continue to have symptoms localizing to the site of injection, even after a normal study. However, the optimal duration between MRI studies is unknown.
- Clinicians should also consider reviewing MRI results with a neuroradiologist because of potential difficulties in interpreting imaging results for these patients.
Fungal Meningitis
For patients with fungal meningitis, what are the risks of more severe outcomes, such as stroke or death?
The estimated risk of stroke or death is likely to be no greater than 0.08% by 42 days after the patient’s last injection. The majority of these patients will have risks of stroke or death that are much lower than the estimates noted here, and their risk will continue to decrease as more time elapses since their last injection.
What are the side effects of being tested on a regular basis for fungal meningitis (lumbar puncture)?
The side effects of lumbar puncture include post-lumbar headache, bleeding, and the theoretical risk that fungi could be transferred from the epidural space into the subarachnoid space of a patient who has received epidural or paraspinal injections with contaminated steroid products. Increasing the number of lumbar punctures will increase the risk of side effects.
Treatment Questions
How should asymptomatic patients who received injections with contaminated steroid medication be managed?
CDC does not recommend initiating antifungal treatment in the absence of diagnostic test results indicating meningitis, spinal/paraspinal infection, or peripheral joint infection in exposed, asymptomatic patients. However, clinicians should remain vigilant for evidence of fungal infection in exposed patients (e.g., continued, worsening or new symptoms at or near the site of their injection) and use an assertive approach for clinical management and follow-up of these patients.
Should patients presenting with arachnoiditis be treated even if this condition is not specifically mentioned in the CDC case definitions for this outbreak?
Yes. The case definition for this outbreak is a surveillance tool developed to assist identifying and reporting cases. The case definition is not intended to guide clinical decision-making and patient management. Patients who present with signs and symptoms of arachnoiditis should be clinically assessed on the basis of clinical judgment and managed accordingly.
What are the side effects of antifungal therapy?
Antifungal treatment with voriconazole carries significant risk of hallucinations and other neurologic side effects, and liver damage. CDC recommends careful discussion of risks and benefits between physicians and their patients. Additional information about side effects can be found under Treatment Guidance.
Can patients continue receiving treatment with steroid injections?
It is not clear if or how additional epidural, paraspinal, or intra-articular steroid injections may increase the risk of fungal infection or contribute to a recurrence of infection in patients who received injections with the contaminated product and who are currently asymptomatic. Steroids are immunosuppressive and it is possible they could increase risk in patients with sub-clinical infection; however, the duration of infection risk resulting from prior exposure to a contaminated steroid product is still unknown. Providers should discuss the need for additional injections with their patients.
Related Recalls
Are other medications from the New England Compounding Center (NECC) associated with infections?
CDC and FDA have identified bacteria and/or fungi in additional products from NECC. Although CDC has received reports of illness in patients who have received these other medications, including some patients who had evidence of meningeal inflammation, CDC and public health officials have no reports of laboratory-confirmed bacterial or fungal meningitis, spinal, or paraspinal infections caused by these products as of October 23, 2013. The available epidemiological and laboratory data do not support evidence of an outbreak of infections linked to usage of non-methylprednisolone acetate NECC products.
However, because it is possible that some of the organisms identified in these products can cause human disease, clinicians should continue to include bacterial and/or fungal infection in the differential diagnosis when evaluating symptomatic patients who were exposed to these medications, including consideration of empiric anti-bacterial and/or antifungal therapy.
For information on other recalled medication from NECC, see Information about Additional Medical Products (non-MPA) From New England Compounding Center FAQs.
Diagnosis Guidance
Diagnosis of Fungal Infection
Thorough and rapid diagnostic evaluation is essential to identify pathogens causing infections in patients who received contaminated steroid injections from the New England Compounding Center. The proper identification of pathogens may have implications for the nature of and duration of antimicrobial therapy.
These instructions are meant to supplement routine laboratory and microbiologic tests deemed necessary by the clinician and should not replace existing diagnostic protocols.
A negative fungal culture or negative fungal polymerase chain reaction (PCR) test from a diagnostic specimen obtained from CSF, a parameningeal site, a joint space, or bone does not rule out infection. Active fungal infection may be present even when these tests are negative.
Diagnosis of Localized Spinal / Paraspinal Infection
The majority of cases in this outbreak, are localized spinal or paraspinal infections (i.e., epidural abscess, phlegmon, discitis, vertebral osteomyelitis, arachnoiditis, or other complications at or near the injection site).
These localized infections may occur on their own or in patients previously diagnosed with fungal meningitis. Although patients with these localized infections frequently have new or worsening back pain, symptoms may be mild or clinically difficult to distinguish from the patient’s baseline chronic pain. Some of these cases have occurred in patients with back pain symptoms at or near baseline. CDC recommends the following protocol:
- In patients with new or worsening symptoms at or near the injection site, physicians should obtain an MRI with contrast of the symptomatic area(s), if not contraindicated. This recommendation also applies to patients being treated for meningitis. In some cases, radiologic evidence of abscess or phlegmon has become apparent on repeat MRI studies performed subsequent to an initially normal imaging procedure. Clinicians should therefore have a low threshold for repeat MRI studies in patients who continue to have symptoms at or near the site of injection, even after a normal study. However, the optimal duration between MRI studies is unknown.
- Clinicians should consider obtaining an MRI with contrast of the symptomatic area(s) in patients with persistent but baseline symptoms following spinal or paraspinal injection of the implicated MPA because the presentation of these spinal or paraspinal infections can be subtle and difficult to distinguish from a patient’s baseline chronic pain.
- CDC has received reports of patients being treated for fungal meningitis who had no previous evidence of localized infection at the site of injection, but who were subsequently found to have evidence of localized infection on imaging studies. Therefore, in patients being treated for meningitis, even in the absence of new or worsening symptoms at or near the injection site, clinicians should strongly consider obtaining an MRI of the injection site approximately 2-3 weeks after diagnosis of meningitis. Early identification of new disease may lead to additional specific interventions (e.g., drainage).
- Patients who have epidural abscess, phlegmon, discitis, vertebral osteomyelitis, or arachnoiditis, should speak with a neurosurgeon to discuss whether surgical management, including debridement, is warranted in addition to antifungal therapy. If a surgical procedure is performed, potentially infected fluid collections should be sampled and sent for microbiologic testing (including fungal smear).
- Any relevant tissue specimens sent for histopathology should be stained and reviewed for infectious agents, including fungi (silver stain). Save specimens to send to state health departments and CDC for further evaluation. If fresh tissue can be saved, freeze at -70°C. See attached guidance for specimen collection and processing
Diagnosis of Central Nervous System Infection Cerebrospinal fluid (CSF)
When possible, collect a large volume of cerebrospinal fluid (CSF), ideally using a different site than was used for the epidural injection. Obtain routine gram stain and bacterial cultures, including aerobic and anaerobic. The priority for remaining CSF specimens is fungal culture, conducted at the local hospital or state lab. When possible, submit a large volume of CSF (minimum 10mL) for fungal culture.
Remaining CSF may be sent to CDC for a polymerse chain reaction (PCR) test. The minimum volume should be 1 mL; 5 mL is preferred. Samples sent to CDC should be unspun samples or freshly collected, unadulterated samples. If only a small volume can be obtained for CDC and the patient meets the case definition, send what you can.
CSF should be sent to CDC only on patients with CSF results showing >5 white blood cells (use traumatic tap correction for WBC), regardless of glucose or protein levels. Specifically for the work-up of possible fungal pathogens:
Attempt to obtain larger volume of CSF to culture for fungi from intraventricular shunts/drains if patient presents with these.
All cultures should be incubated for at least 2 weeks prior to discarding.
Other tests
In addition to routine blood cultures, consider obtaining fungal blood cultures. Other potentially infected fluid collections should be sampled (e.g., aspiration of epidural abscess) and sent for microbiologic testing as described above for CSF specimens (including fungal smear). Any relevant tissue specimens sent for histopathology should be stained and reviewed for infectious agents, including fungi (silver stain). Save specimens to send to state health departments and CDC for further evaluation. If fresh tissue can be saved, freeze at -70°C.
Send available autopsy specimens to CDC for further evaluation.
Diagnosis of Joint Infection
Perform arthrocentesis to obtain synovial fluid for analysis. If initial presentation or diagnostic testing suggests severe infection, consider arthroscopy, in consultation with an orthopedic surgeon, to obtain synovial fluid and tissue and to perform lavage and/or debridement.
The following testing is recommended:
- Send synovial fluid for fungal culture, in addition to cell count and differential, routine Gram stain, bacterial cultures and examination for crystals.
- Send tissue specimens for histopathology and review for infectious agents, including fungi (silver stain).
- Send remaining synovial fluid (ideally ≥1 mL) and tissue specimens to CDC for further testing, including PCR of synovial fluid. If fresh tissue can be saved, freeze at -70°C. See attached guidance for specimen collection and processing.
Radiographic imaging studies, such as MRI, may be considered to evaluate for osteomyelitis. Imaging may be particularly important in the evaluation of joint spaces such as the sacroiliac joint, where osteomyelitis may be more common and from which obtaining a diagnostic specimen of synovial fluid may be more difficult. Similarly, for patients in whom complaints of back pain are worrisome for the development of discitis/vertebral osteomyelitis, imaging should be performed. In some of these cases, repeat imaging may be required as the progression of the infection may be slow and imaging findings suggestive or diagnostic of osteomyelitis may not be evident for weeks following initial presentation. If osteomyelitis is suspected, arthroscopic biopsy and debridement in consultation with an orthopedic surgeon is recommended. Tissue specimen should undergo testing and submission to CDC as described above.
Specimen Shipping Information
Please contact your state health department and state public health laboratory to coordinate shipment of specimens to CDC for further testing.
CDC's PCR test for detection of fungal DNA
CDC’s fungal PCR test At-A-Glance
- Limit of detection: 1 picogram DNA per ml
- Diagnostic sensitivity of PCR: 29%
- Diagnostic sensitivity of fungal culture: 14%
- Diagnostic specificity of PCR: 100%
CDC has developed a novel test using PCR and DNA sequencing to detect fungal DNA in cerebrospinal fluid (CSF), other body fluids, and tissues from patients in this outbreak. To assess the performance of this PCR test, CDC tested 627 samples from 413 case-patients. The main results of the validation study are as follows:
- The PCR test detected Exserohilum rostratum DNA in 123 samples from 114 case-patients (28% of the 413 case-patients for whom samples were available). The test also detected Cladosporium sp. DNA in one case-patient.
- PCR was more sensitive than culture. Of 139 case-patients who had a specimen tested by both PCR and culture, E. rostratum DNA was detected by PCR in 41 (29%) but was only recovered from culture in 19 (14%). In total, 33% of case-patients had fungi detected in specimens by either culture or PCR.
- There were no false-positive PCR results (100% specificity). Among 136 specimens from patients who did not meet the case definition, all were negative.
The full results of the study performed to validate this PCR test are available here. The results suggest that the test is a useful tool to quickly detect fungi in body fluids and tissues, and it can detect fungal DNA in specimens that may not have shown any fungus in culture.
However, PCR for fungal detection is still a research test that has not been cleared or approved by the FDA and should not be used for diagnosis, treatment, or assessment of patient health or management. In addition, for both PCR and culture, a negative result does not rule out infection.
Treatment Guidance
Interim Treatment Guidance for Central Nervous System and Parameningeal Infections Associated with Injection of Contaminated Steroid Products
Rationale
The following guidance is for treatment of adult patients with central nervous system (CNS) infections (including meningitis, stroke, and arachnoiditis [1]) and/or parameningeal infections (epidural or paraspinal abscess, discitis or osteomyelitis, and sacroiliac infection) associated with injections of contaminated steroid products from the New England Compounding Center. Parameningeal infections include any infections at or near the site of injection into the spinal or paraspinal area, including the sacroiliac joint.
These recommendations are based upon evidence that Exserohilum rostratum (a brown-black mold) is the predominant pathogen in this outbreak, and expert opinion and published literature indicating that voriconazole may be effective in treating infections due to brown-black molds as well as infections due to Aspergillus species. Recommendations are also based on considerations related to the anatomic site of infection, the pharmacokinetics of antifungal agents, and the clinical course, including complications (e.g., arachnoiditis, mycotic aneurysms, development of new CNS or epidural abscesses or masses, and stroke) that have been found during the course of treatment among some patients with fungal infections associated with this outbreak. CDC arrived at this guidance through consultation with national experts and clinicians managing patients associated with this outbreak.
Infectious Diseases Physician Consultation
Consult an infectious diseases physician to assist with patient diagnosis, management, and follow-up. Optimal therapy for these infections has not been established and may be complex and prolonged. Expert consultation is particularly important because overall clinical experience is so limited. CDC has established a clinical consultant network for physicians which can be reached by calling CDC info: 1-800-CDC-INFO.
Considerations for Antifungal Therapy in Patients with CNS and/or Parameningeal Infection
The diagnosis of CNS and/or parameningeal infections should be suspected in patients who received injections with one of the three implicated lots of methylprednisolone and who has either increased pain at the injection site, MRI evidence of inflammation or unexplained pleocytosis in the CSF.
Antifungal therapy should be initiated after collecting appropriate specimens (CSF, abscess fluid, tissue) for culture and PCR, in addition to routine empiric treatment protocols for other pathogens, until the etiology of the patient’s CNS and/or parameningeal infection has been identified. Specific tests to identify fungus are often negative in patients with fungal infection. Confirmation of fungus by culture, PCR, or pathology has been found in approximately 30% of cases to date.
- Providers should give voriconazole, preferably at an initial dose of 6 milligrams per kilogram (mg/kg) every 12 hours [2]. This dose is recommended in order to ensure rapid and adequate penetration of voriconazole into the CNS, as well as allowing for blood levels to come up quickly.
- A blood specimen for serum voriconazole trough level measurement should be collected on the fifth day of voriconazole treatment, and the dose of voriconazole should be adjusted based on this trough level, aiming for a trough level range of 2 to 5 micrograms per milliliter (mcg/ml). Serum voriconazole trough levels greater than 5 mcg/ml should be avoided because of the risk of neurotoxicity and other drug-related adverse events. There is however substantial overlap between levels in patients for both toxic and nontoxic side effects, so clinicians should monitor patients and not just blood levels.
- Regular monitoring of serum voriconazole trough levels once per week should occur for the initial 4-6 weeks of voriconazole treatment and when dose adjustments are made. Dose adjustments should be made as needed to maintain serum voriconazole trough levels within the range of 2 to 5 mcg/ml.
- In most cases it is suggested that patients should be started on intravenous (IV) voriconazole. If the provider wants to transition patients initially treated with IV voriconazole to oral voriconazole, this should be done only after a patient is clinically stable or improving, as long as no contraindications to oral therapy exist.
- Although it is recommended that most patients with CNS or parameningeal infections be started on IV voriconazole, patients with mild disease who are able to take oral voriconazole as prescribed and who are able to be monitored closely may be started on oral voriconazole at the provider’s discretion. Patients on oral voriconazole should be treated with a dose of 6 mg/kg every 12 hours given on an empty stomach, with monitoring of serum voriconazole trough levels as above and dose adjustment as necessary. The target range for serum voriconazole trough levels (2 to 5 mcg/ml) is readily achievable using the oral form of the drug, but may require a slightly higher dose and may take longer to achieve if gastrointestinal intolerance or poor absorption are encountered.
- Providers should carefully consider and manage potential voriconazole drug interactions in all patients. Potential adverse effects of voriconazole include (but are not limited to) hepatic toxicity and neurotoxicity. Liver function tests should be closely monitored. Minor elevations of transaminases and alkaline phosphatase are common, but severe hepatotoxicity can also occur. Visual hallucinations are not rare and often respond to dose reduction and reassurance. Discontinuance of voriconazole should not be automatic. Although some side effects of voriconazole are dose-related, others appear to not be related to high voriconazole serum concentrations. Frequently, patients who have levels within the therapeutic range complain of a “foggy” feeling, being unable to make decisions, and forgetfulness. Nausea, anorexia, and fatigue are commonly experienced. Chapped lips are very common, some patients have noticed alopecia. Sun sensitivity while on voriconazole can be severe and patients who have received many months of voriconazole may be at increased risk of skin cancer. Another rare complication of long term voriconazole is periostitis, which can present as bone pain and elevated alkaline phosphatase.
- Providers should strongly consider giving liposomal amphotericin B in addition to voriconazole to patients who present with severe disease and patients who do not improve or who experience clinical deterioration or who manifest new sites of disease activity while on voriconazole monotherapy. Liposomal amphotericin B may also be considered as an alternative to voriconazole in patients who are unable to tolerate voriconazole.
- When used, liposomal amphotericin B should be given at a dose of 5 to 6 mg/kg IV daily. The liposomal preparation of amphotericin B [AmBisome®] is preferred over other lipid formulations because of better CNS penetration. Nephrotoxicity is a common complication of amphotericin B therapy, including therapy with lipid formulations of amphotericin B. Kidney function and electrolytes should be monitored closely in patients receiving any amphotericin B formulation. Administration of 1 liter of normal saline IV prior to amphotericin B infusion may be considered to minimize risk of nephrotoxicity. Providers and patients should also be aware of and monitor for other potential adverse effects of amphotericin B formulations.
- Higher doses of liposomal amphotericin B (7.5 mg/kg IV daily) may be considered for patients who are not improving, recognizing the potential for increased nephrotoxicity on this higher dose.
- The routine use of intrathecal amphotericin B formulations is not recommended, due to limited data on their use and associated toxicities.
Considerations Regarding Other Antifungal Medications
It is important to continue to use voriconazole or amphotericin B as primary treatment. Side effects, such as hallucinations with voriconazole and minor elevations in creatinine with amphotericin are to be expected in some patients and may not necessitate discontinuation of those drugs. If such mild to moderate side effects can be managed by adjusting doses, continuation of first-line agents is preferred. Some patients may be unable to tolerate voriconazole (due to hepatotoxicity or other severe side effects) or amphotericin B (due to severe nephrotoxicity or other serious adverse reactions). For these patients and those with other contraindications to voriconazole and amphotericin B, some infectious diseases clinicians have used other anti-fungal agents such as posaconazole or itraconazole. These medications have variable pharmacokinetics within the CNS and their effectiveness for treatment of infections associated with this outbreak is not established. However, use of these drugs deserves consideration when first line therapies cannot be safely administered to the patient. Decisions to change anti-fungal therapy should be made in consultation with an infectious disease physician experienced in the treatment of fungal infections.
Considerations for Surgical Management of Parameningeal Disease
Individual patient management decisions, including surgical debridement and drainage of abscess fluid, should be made in consultation with surgical teams and infectious diseases physicians. Some clinical teams have performed surgical drainage rapidly after diagnosis as an adjunct to antifungal therapy on patients with localized abscesses and phlegmons at the injection site. Whether this clinical approach is superior to antifungal therapy alone is currently unknown.
Considerations Regarding Duration of Antifungal Treatment for CNS Infections With and Without Parameningeal Disease
Adequate duration of antifungal treatment is unknown, and patients likely will require prolonged therapy tailored by the clinical response to treatment. Individual patient management decisions, including choice of long-term antifungal treatment regimens, should be made in consultation with infectious diseases physicians experienced in the treatment of fungal infections. The appropriate duration of therapy will likely vary substantially depending upon individual patient circumstances as well as severity of CNS disease. In general, patients with meningitis alone will likely need a minimum of three months of antifungal treatment. Patients with more severe CNS disease including complications such as arachnoiditis or stroke, those with persistent CSF abnormalities, or those who have underlying immunosuppression will likely need to continue antifungal treatment for six months to one year. Follow-up monitoring after completion of therapy is important to detect potential relapse of infection (see section Considerations for Monitoring Clinical Status After Cessation of Anti-fungal Treatment).
Considerations Regarding Duration of Antifungal Treatment for Parameningeal Infections not Associated with CNS Disease
Adequate duration of antifungal treatment is unknown, and patients likely will require prolonged therapy tailored by the clinical response to treatment. Appropriate management of these infections will likely vary substantially depending upon individual patient circumstances, a minimum of three to six months of antifungal treatment should be considered in patients with parameningeal infection. Treatment will likely need to continue for at least six months or longer in patients with more severe disease (e.g., discitis or osteomyelitis), underlying immunosuppression, and other complications that may not be amenable to surgical treatment. Follow-up monitoring after completion of therapy is important to detect potential relapse of infection (see section Considerations for Monitoring Clinical Status After Cessation of Anti-fungal Treatment).
Considerations for Monitoring Clinical Status After Cessation of Antifungal Treatment
Because adequate duration of treatment and the potential for relapse are unknown, close monitoring of patients in whom antifungal therapy has been discontinued is essential. Patients with recurrent symptoms or worsening pain should be evaluated promptly. There should be a low threshold for performing lumbar puncture or imaging to diagnose relapsed infection. Radiologic abnormalities may persist for several months after clinical symptoms have resolved.
Osteoarticular Infections
Rationale
This is guidance for treatment of adult patients with osteoarticular infections associated with intra-articular injections of contaminated steroid products from the New England Compounding Center.
These recommendations are based upon evidence that Exserohilum rostratum (a brown-black mold) is the predominant pathogen in this outbreak, and expert opinion and published literature indicating that voriconazole may be effective in treating infections due to brown-black molds, as well as infections due to Aspergillus species. Recommendations are also based on considerations related to the anatomic site of infection and pharmacokinetics of antifungal agents. CDC consulted with national experts about treatment options for fungal osteoarticular infections in patients associated with this outbreak.
Infectious Diseases Physician Consultation
Consult an infectious diseases physician to assist with patient diagnosis, management, and follow up, which may be complex and prolonged.
Diagnostic Considerations
Thorough diagnostic evaluation is essential, and should include collection of synovial fluid and/or synovial tissue prior to initiation of treatment. Samples should be sent for fungal culture, molecular testing, and histopathological examination, in addition to the standard tests for bacterial infection and crystal-induced disease.
Physicians should use their best judgment in regard to imaging the osteoarticular structure(s) that may be infected. For large joints, such as knees, if there is a consideration of adjacent osteomyelitis, then imaging should be performed. Imaging may be particularly important in the evaluation of joint spaces such as the sacroiliac joint, where osteomyelitis may be more common and from which obtaining a diagnostic specimen of synovial fluid may be more difficult. Similarly, for patients in whom complaints of back pain are worrisome for the development of discitis/vertebral osteomyelitis, imaging should be performed. In some of these cases, repeat imaging may be required as the progression of the infection may be slow and imaging findings suggestive or diagnostic of osteomyelitis may not be evident for two weeks or more following initial presentation.
Note that a negative fungal culture or negative fungal polymerase chain reaction (PCR) test from a diagnostic specimen obtained from a joint space or bone does not rule out infection. Active fungal infection may be present even when these tests are negative.
Empiric Antifungal Therapy
- Routine empiric antibacterial therapy should be used according to the judgment of the physician while awaiting results of diagnostic studies.
- In clinically stable patients with peripheral joint infection, it may be reasonable to wait 48-72 hours before initiating empiric antifungal therapy, to allow time for identification of alternative diagnoses (e.g., bacterial arthritis, crystal-induced arthritis, etc.). This decision should be made at the discretion of the provider.
- When empiric antifungal therapy is initiated, the following regimen is suggested until the etiology of the patient’s septic arthritis has been identified:
- For discitis, vertebral osteomyelitis, and epidural abscess give voriconazole at a dose of 6 milligrams per kilogram (mg/kg) every 12 hours[2]. For osteoarticular infections that do not involve the spine, give voriconazole, beginning with a loading dose of 6 mg/kg every 12 hours for two doses, followed by 4 mg/kg every 12 hours[2].
- A blood specimen for serum voriconazole trough level measurement should be collected on the 5th day of voriconazole treatment, and the dose of voriconazole should be adjusted based on this trough level, aiming for a trough level range of 2 to 5 micrograms per milliliter (mcg/ml). Serum voriconazole trough levels greater than 5 mcg/ml should be avoided because of the risk of neurotoxicity and other drug-related adverse events.
- Regular monitoring of serum voriconazole trough levels once per week should occur for the initial four to six weeks of voriconazole treatment and when dose adjustments are made. Dose adjustments should be made as needed to maintain serum voriconazole trough levels within the range of 2 to 5 mcg/ml.
- Patients with more severe osteoarticular infection, clinical instability, discitis, vertebral osteomyelitis, or epidural abscess should be started on voriconazole IV. If the provider wants to transition patients initially treated with IV voriconazole to oral voriconazole, this should be done only after a patient is clinically stable or improving, as long as no contraindications to oral therapy exist.
- Patients with mild osteoarticular infection not involving the spine who are able to take oral voriconazole as prescribed and who are able to be monitored closely may be started on oral voriconazole at the provider’s discretion. Patients on oral voriconazole should be treated with a loading dose of 6 mg/kg every 12 hours for two doses, followed by 4 mg/kg every 12 hours, with monitoring of serum voriconazole trough levels as above and dose adjustment as necessary. The target range for serum voriconazole trough levels (2 to 5 mcg/ml) is readily achievable using the oral form of the drug, but may require a slightly higher dose and may take longer to achieve if unforeseen problems with gastrointestinal intolerance or poor absorption are encountered.
- Providers and patients should be aware of and monitor for potential adverse effects of voriconazole, including (but not limited to) hepatic toxicity and neurotoxicity. Liver function tests should be closely monitored. The occurrence of hallucinations may be an indication of neurotoxicity and elevated serum voriconazole levels, and should prompt collection of a blood specimen for serum voriconazole trough level measurement and voriconazole dose adjustment.
- Providers should carefully consider and manage the potential for voriconazole drug interactions in all patients.
- A lipid formulation of amphotericin B at a dose of 5 mg/kg IV daily should be considered in addition to voriconazole in patients with severe osteoarticular infection and/or patients with clinical instability. Nephrotoxicity is a common complication of amphotericin B therapy, including therapy with lipid formulations of amphotericin B. Kidney function and electrolytes should be monitored closely in patients receiving any amphotericin B formulation. Administration of 1 liter of normal saline IV prior to amphotericin B infusion may be considered to minimize risk of nephrotoxicity. Providers and patients should also be aware of and monitor for other potential adverse effects of amphotericin B formulations.
- Alternate therapies to consider for patients who are unable to tolerate treatment with voriconazole include lipid formulations of amphotericin B, posaconazole, and itraconazole. Therapeutic drug monitoring should be performed for patients treated with posaconazole or itraconazole. Consultation with an infectious diseases physician should be sought regarding dosages and monitoring of these agents. Fluconazole should NOT be used.
Other Treatment Considerations
Arthroscopy with joint lavage and debridement should be considered in consultation with an orthopedic surgeon, or a neurosurgeon in the case of vertebral osteomyelitis.
Considerations Regarding Duration of Treatment
Adequate duration of antifungal treatment is unknown, and it is likely that patients will require prolonged therapy tailored by the clinical response to treatment. Individual patient management decisions, including choice of long-term antifungal treatment regimens, should be made in consultation with infectious diseases physicians experienced in the treatment of fungal infections. While adequate duration of therapy is unknown and will likely vary substantially depending upon individual patient circumstances, a minimum of 3 months of antifungal treatment should be considered. Treatment may need to continue for longer than 3 months in patients with more severe disease, bone infection, underlying immunosuppression, etc. Clinicians should be vigilant for potential relapse of infection after completion of therapy.
- 1 Symptoms consistent with meningitis in addition to CSF WBC greater than 5 in a non-traumatic lumbar puncture. If a traumatic lumbar puncture is suspected, a corrected CSF WBC count can be calculated by subtracting one WBC for every 500 red blood cells (RBC) present in the CSF.
- 2 Dose adjustments may be needed for certain patients, including (but not necessarily limited to): children (who often need a higher dose) and patients with hepatic impairment (who may need a lower dose). Dosing of voriconazole in obese patients should be discussed with an infectious diseases physician. Oral voriconazole should be taken at least one hour before or after a meal. Consult an infectious diseases specialist and refer to the manufacturer’s instructions.
Resources
For additional information on antifungal drugs
- Voriconazole package insert
- Liposomal amphotericin B package insert [PDF – 27 pages]
- Amphotericin B lipid complex package insert [PDF – 2 pages]
- Other sources for drug information:
http://www.nlm.nih.gov/medlineplus/druginformation.html - FDA information on voriconazole IV and liposomal amphotericin B availability
Additional Resources
Clinical Calls and Communications
- 2013
- 2012
Health Alert Network Updates
- CDC Health Alert Network (HAN): Update: Notice to Clinicians: Continued Vigilance Urged for Fungal Infections Among Patients Who Received Contaminated Steroid Injections, March 4, 2013
- CDC Health Alert Network (HAN): Update: Multistate Outbreak of Fungal Infections among Persons Who Received Injections with Contaminated Medication, December 20, 2012
- CDC Health Alert Network (HAN): Update: Additional Contamination Identified in Medical Products from New England Compounding Center, December 3, 2012
- CDC Health Alert Network (HAN): Update: Multistate Outbreak of Fungal Meningitis and Other Infections Associated with Contaminated Steroid Medication , November 20, 2012
- CDC Health Alert Network (HAN): Contamination Identified in Additional Medical Products from New England Compounding Center, November 1, 2012
- CDC Health Alert Network (HAN): Voluntary Recall of All Ameridose Medical Products, November 1, 2012
- CDC Health Alert Network (HAN): Issuance of Guidance on Management of Asymptomatic Patients Who Received Epidural or Paraspinal Injections with Contaminated Steroid Products, October 23, 2012
- CDC Health Alert Network (HAN): Update: Multistate Outbreak of Fungal Meningitis and Joint Infections Associated with Contaminated Steroid Medications, October 17, 2012
- CDC Health Alert Network (HAN): Multistate Outbreak of Meningitis and Stroke Associated with Potentially Contaminated Steroid Medication, October 8, 2012
- CDC Health Alert Network (HAN): Meningitis and Stroke Associated with Potentially Contaminated Product, October 4, 2012
CDC Morbidity and Mortality Weekly Report (MMWR)
- MMWR: Multistate Outbreak of Fungal Infection Associated with Injection of Methylprednisolone Acetate Solution from a Single Compounding Pharmacy — United States, 2012
- MMWR: Multistate Fungal Meningitis Outbreak — Interim Guidance for Treatment
CDC Resources
- Frequently Asked Questions for Patients
- CDC Digital Press Kit with Laboratory Photos and Footage
- CDC Fungal Diseases
- CDC Information About Additional Medical Products (non-MPA) From New England Compounding Center
- CDC Information About Voluntary Recall of All Ameridose Medical Products
- CDC and Public Health Response to the 2012 Fungal Meningitis and other Infections Outbreak: Statement of Beth Bell, MD, MPH [PDF – 8 pages]
Select Publications
- Bell BP, Khabbaz RF. Responding to the Outbreak of Invasive Fungal Infections: The Value of Public Health to Americans. JAMA. 2013;():1-2. doi:10.1001/jama.2013.526.
- Smith RM, Schaefer MK, Kainer, MA, et al. Fungal infections associated with contaminated methylprednisolone injections – Preliminary Report. New Engl J Med 2012 Dec.
- Kainer MA, Reagan DR, Nguyen DB, et al. Fungal infections associated with contaminated methylprednisolone in Tennessee. New Engl J Med 2012 Nov.
- Pettit AC, Kropski JA, Castilho JL, et al. The index case for the fungal meningitis outbreak in the United States. New Engl J Med 2012 Oct.
U.S. Food and Drug Administration (FDA)
- Page last reviewed: October 29, 2015
- Page last updated: October 29, 2015
- Content source:


 ShareCompartir
ShareCompartir