Meningococcal Disease
On this Page
Neisseria meningitidis
- Severe acute bacterial infection
- Cause of meningitis, sepsis, and focal disease (e.g. pneumonia and arthritis)
- Epidemic disease in sub-Saharan Africa
- Quadrivalent polysaccharide vaccine licensed in 1981
- Conjugate vaccine licensed in U.S. 2005
- Aerobic gram-negative bacteria
- 13 distinct polysaccharide capsules have been described
- Almost all invasive disease caused by serogroups A, B, C, Y, and W
- Relative importance of serogroups depends on geographic location and other factors (e.g. age)
Meningococcal disease is an acute, potentially severe illness caused by the bacterium Neisseria meningitidis. Illness believed to be meningococcal disease was first reported in the 16th century. The first definitive description of the disease was by Vieusseux in Switzerland in 1805. The bacterium was first identified in the spinal fluid of patients by Weichselbaum in 1887.
Neisseria meningitidis is a leading cause of bacterial meningitis and sepsis in the United States. It can also cause focal disease, such as pneumonia and arthritis. N. meningitidis is also a cause of epidemics of meningitis and bacteremia in sub-Saharan Africa. The World Health Organization has estimated that meningococcal disease was the cause of 171,000 deaths worldwide in 2000.
The first monovalent (serogroup C) polysaccharide vaccine was licensed in the United States in 1974. A quadrivalent polysaccharide vaccine was licensed in 1981. Monovalent serogroup C meningococcal conjugate vaccine has been licensed in United Kingdom since 1999 and has had a major impact on the incidence of serogroup C meningococcal disease. Quadrivalent conjugate vaccines were first licensed in the United States in 2005. A bivalent conjugate combination vaccine (with Hib) was licensed in the United States in 2012, and two serogroup B recombinant vaccines were licensed in early 2015.
Neisseria meningitidis
N. meningitidis, or meningococcus, is an aerobic, gram-negative diplococcus, closely related to N. gonorrhoeae, and to several nonpathogenic Neisseria species, such as N. lactamica. The organism has both an inner (cytoplasmic) and an outer membrane, separated by a cell wall. The outer membrane contains several protein structures that enable the bacteria to interact with the host cells as well as perform other functions.
Meningococcal Disease Pathogenesis
- Organism colonizes nasopharynx
- In some persons organism invades bloodstream and causes infection at distant site
- Antecedent URI may be a contributing factor
Neisseria meningitidis Clinical Features
- Incubation period 3-4 days (range 2-10 days)
- Abrupt onset of fever, meningeal symptoms, hypotension, and rash
- Fatality rate 10%-15%, up to 40% in meningococcemia
Meningococcal Meningitis
- Most common presentation of invasive disease
- Result of hematogenous dissemination
- Clinical findings
- fever
- headache
- stiff neck
Meningococcemia
- Bloodstream infection
- May occur with or without meningitis
- Clinical findings
- fever
- petechial or purpuric rash
- hypotension
- shock
- acute adrenal hemorrhage
- multiorgan failure
The outer membrane is surrounded by a polysaccharide capsule that is necessary for pathogenicity because it helps the bacteria resist phagocytosis and complement-mediated lysis. The outer membrane proteins and the capsular polysaccharide make up the main surface antigens of the organism.
Meningococci are classified by using serologic methods based on the structure of the polysaccharide capsule. Thirteen antigenically and chemically distinct polysaccharide capsules have been described. Some strains, often those found to cause asymptomatic nasopharyngeal carriage, are not groupable and do not have a capsule. Almost all invasive disease is caused by one of five serogroups: A, B, C, W, and Y. The relative importance of each serogroup depends on geographic location, as well as other factors, such as age. For instance, serogroup A has historically been a major cause of disease in sub-Saharan Africa but is rarely isolated in the United States.
Meningococci are further classified on the basis of certain outer membrane proteins. Molecular subtyping using specialized laboratory techniques (e.g., pulsed-field gel electrophoresis) can provide useful epidemiologic information.
Pathogenesis
Meningococci are transmitted by droplet aerosol or secretions from the nasopharynx of colonized persons. The bacteria attach to and multiply on the mucosal cells of the nasopharynx. In a small proportion (less than 1%) of colonized persons, the organism penetrates the mucosal cells and enters the bloodstream. The bacteria spread by way of the blood to many organs. In about 50% of bacteremic persons, the organism crosses the blood–brain barrier into the cerebrospinal fluid and causes purulent meningitis. An antecedent upper respiratory infection (URI) may be a contributing factor.
Clinical Features
The incubation period of meningococcal disease is 3 to 4 days, with a range of 2 to 10 days.
Meningitis is the most common presentation of invasive meningococcal infection (meningococcal disease) and results from hematogenous dissemination of the organism. Meningeal infection is similar to other forms of acute purulent meningitis, with sudden onset of fever, headache, and stiff neck, often accompanied by other symptoms, such as nausea, vomiting, photophobia (eye sensitivity to light), and altered mental status. Meningococci can be isolated from the blood in up to 75% of persons with meningitis.
Meningococcal sepsis (bloodstream infection or meningococcemia) occurs without meningitis in 5% to 20% of invasive meningococcal infections. This condition is characterized by abrupt onset of fever and a petechial or purpuric rash, often associated with hypotension, shock, acute adrenal hemorrhage, and multiorgan failure.
Less common presentations of meningococcal disease include pneumonia (5% to 15% of cases), arthritis (2%), otitis media (1%), and epiglottitis (less than 1%).
The case-fatality ratio of meningococcal disease is 10% to 15%, even with appropriate antibiotic therapy. The case-fatality ratio of meningococcemia is up to 40%. As many as 20% of survivors have permanent sequelae, such as hearing loss, neurologic damage, or loss of a limb.
Risk factors for the development of meningococcal disease include deficiencies in the terminal common complement pathway, functional or anatomic asplenia, and underlying chronic disease. Persons with HIV infection are probably at increased risk for meningococcal disease. Certain genetic factors (such as polymorphisms in the genes for mannose-binding lectin and tumor necrosis factor) may also be risk factors.
Neisseria meningitidis Risk Factors for Invasive Disease
- Host factors
- deficiencies in the terminal common complement pathway
- functional or anatomic asplenia
- certain genetic factors
- Environmental factors
- antecedent viral infection
- household crowding
- active and passive smoking
- occupational (microbiologists)
Meningococcemia Disease Laboratory Diagnosis
- Bacterial culture
- Gram stain
- Non-culture methods
- PCR
- antigen detection in CSF
- serology
Household crowding, and both active and passive smoking are associated with increased risk. Persons with antecedent viral infection are also at increased risk. Early studies in the United States demonstrated that blacks and persons of low socioeconomic status were at higher risk for meningococcal disease than other persons; however, race and low socioeconomic status are likely markers for differences in factors such as smoking and household crowding rather than risk factors. As disease incidence has decreased, differences by race have also decreased, and no difference in disease incidence exists now between blacks and whites. During outbreaks, bar or nightclub patronage and alcohol use have also been associated with higher risk for disease.
Cases of meningococcal disease, including at least two fatal cases, have been reported among microbiologists. These persons have worked with N. meningitidis isolates rather than patient specimens.
Laboratory Diagnosis
Meningococcal disease is typically diagnosed by isolation of N. meningitidis from a normally sterile site. However, sensitivity of bacterial culture may be low, particularly when performed after initiation of antibiotic therapy. A Gram stain of cerebrospinal fluid (CSF) showing gram-negative diplococci strongly suggests meningococcal meningitis. Real-time polymerase chain reaction (rt-PCR) detects DNA of meningococci in blood, cerebrospinal fluid, or other clinical specimens. Although culture remains the criterion standard for diagnosis of meningococcal disease in the United States, PCR is useful for detection of N. meningitidis from clinical samples in which the organism could not be detected by culture methods, such as when a patient has been treated with antibiotics before obtaining a clinical specimen for culture.
Kits to detect polysaccharide antigen in cerebrospinal fluid are rapid and specific, but false-negative results are common, particularly in serogroup B disease. Antigen tests of urine or serum are unreliable.
Serologic testing (e.g., enzyme immunoassay) for antibodies to polysaccharide may be used as part of the evaluation if meningococcal disease is suspected, but should not be used to establish the diagnosis.
Top of PageMedical Management
Neisseria meningitidis Medical Management
- Empiric antibiotic treatment after appropriate cultures are obtained
- Treatment with penicillin alone recommended after confirmation of N. meningitidis
Meningococcal Disease Epidemiology
- Reservoir
- human
- Transmission
- respiratory droplets
- Temporal pattern
- peaks in late winter and early spring
- Communicability
- Generally limited
The clinical presentation of meningococcal meningitis is similar to other forms of bacterial meningitis. Consequently, empiric therapy with broad-spectrum antibiotics (e.g., third-generation cephalosporin, vancomycin) should be started promptly after appropriate cultures have been obtained.
Many antibiotics are effective for N. meningitidis infection, including penicillin. Few penicillin-resistant strains of meningococcus have been reported in the United States. Once N. meningitidis infection has been confirmed, penicillin alone is recommended.
Epidemiology
Occurrence
Meningococcal disease occurs worldwide in both endemic and epidemic form.
Reservoir
Humans are the only natural reservoir of meningococcus. As many as 10% of adolescents and adults are asymptomatic transient carriers of N. meningitidis, most strains of which are not pathogenic (i.e., strains that are not groupable).
Transmission
Primary mode is by respiratory droplet spread or by direct contact.
Temporal Pattern
Meningococcal disease occurs throughout the year, however, the incidence is highest in the late winter and early spring.
Communicability
The communicability of N. meningitidis is generally limited. In studies of households in which a case of meningococcal disease has occurred, only 3%–4% of households had secondary cases. Most households had only one secondary case. Estimates of the risk of secondary transmission are generally 2–4 cases per 1,000 household members at risk. However, this risk is 500–800 times that in the general population.
Secular Trends in the United States
Meningococcal Disease - United States, 1972-2012*
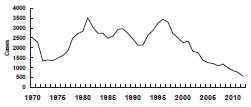
*all serogroups
During 2005-2011, an estimated 800-1,200 cases of meningococcal disease occurred annually in the United States, representing an incidence of 0.3 cases per 100,000 population. Incidence has declined annually since a peak of disease in the late 1990s. Since 2005, declines have occurred among all age groups and in all vaccine-contained serogroups. In addition, incidence of disease attributable to serogroup B, a serogroup not included in the quadrivalent vaccine, declined for reasons that are not known. Serogroups B, C, and Y are the major causes of meningococcal disease in the United States, each being responsible for approximately one third of cases. The proportion of cases caused by each serogroup varies by age group. Approximately 60% of disease among children aged 0 through 59 months is caused by serogroup B, for which no conjugate vaccine is licensed or available in the United States. Serogroups C, W, or Y, which are included in vaccines available in the United States, cause 73% of all cases of meningococcal disease among persons 11 years of age or older.
The incidence of serogroups C and Y, which represent the majority of cases of meningococcal disease preventable by the conjugate vaccines, are at historic lows. However, a peak in disease incidence among adolescents and young adults 16 to 21 years of age has persisted, even after routine vaccination of adolescents was recommended in 2005. From 2000–2004 to 2005–2009, the estimated annual number of cases of serogroups C and Y meningococcal disease decreased 74% among persons aged 11 through 14 years but only 27% among persons aged 15 through 18 years.
Meningococcal Outbreaks in the United States
- Outbreaks account for less than 2% of reported cases
- Most recent outbreaks caused by serogroup C and B
During 2006-2010 (i.e., in the first 5 years after routine use of meningococcal vaccine was recommended) CDC received reports of approximately 30 cases of serogroups C and Y meningococcal disease among persons who had received the vaccine. The case-fatality ratio was similar among persons who had received vaccine compared with those who were unvaccinated. Of the 13 reports of breakthrough disease for which data on underlying conditions were available, four persons had underlying conditions or behaviors associated with increased risk for bacterial infections, including 1) Type 1 diabetes mellitus; 2) current smoking; 3) history of bacterial meningitis and recurrent infections; and 4) aplastic anemia, paroxysmal nocturnal hemoglobinuria, and receipt of eculizumab (which blocks complement protein C5).
In the United States, meningococcal outbreaks account for less than 2% of reported cases (98% of cases are sporadic). However, outbreaks of meningococcal disease continue to occur. During 2010, 2 serogroup C and 2 serogroup B outbreaks were reported to CDC. Cases associated with all reported outbreaks accounted for 108 (1.5%) of the 7,343 cases reported to CDC during 2005-2011. See additional information on the evaluation and management of meningococcal outbreaks [32 pages].
Meningococcal Polysaccharide Vaccine (MPSV4)
- Menomune (Sanofi Pasteur)
- Quadrivalent polysaccharide vaccine (A, C, W, Y)
- Administered by subcutaneous injection
- 10-dose vial contains thimerosal as a preservative
Meningococcal Conjugate Vaccines (MenACWY)
- Menactra (Sanofi Pasteur)
- Menveo (Novartis)
- MenHibrix (GlaxoSmithKline)
Routine MenACWY Vaccination Recommendations
- Administer either MenACWY at age 11 or 12 years with a booster dose at 16 years of age
- Administer 1 dose at age 13 through 15 years if not previously vaccinated
- For persons vaccinated at age 13 through 15 years a 1-time booster dose should be administered, preferably at 16 through 18 years
- Healthy persons who receive their first dose of meningococcal conjugate vaccine at or after age 16 years do not need a booster dose
- Routine vaccination not recommended after age 21 years for healthy persons who are not at increased risk of exposure
- A booster dose is not recommended for healthy persons 22 years of age and older even if the first dose was administered at 11-15 years of age
Historically, large epidemics of serogroup A meningococcal disease occur in the African “meningitis belt,” an area that extends from Ethiopia to Senegal. Rates of endemic meningococcal disease in this area are several times higher than in industrialized countries. In each epidemic, tens of thousands of cases and thousands of deaths may occur. Approximately 350 million people are at risk. The phased introduction in meningitis belt countries of MenAfriVac, a novel serogroup A meningococcal conjugate vaccine which is being implemented through preventive national campaigns of all individuals 1-29 years of age, holds great promise to end epidemic meningitis as a public health concern by 2016.
Meningococcal Vaccine
Characteristics
Meningococcal Polysaccharide Vaccine
The first meningococcal polysaccharide vaccine (MPSV4) was licensed in the United States in 1974. The current quadrivalent A, C, W, Y polysaccharide vaccine (Menomune, Sanofi Pasteur) was licensed in 1981. Each dose consists of 50 mcg of each of the four purified bacterial capsular polysaccharides. The vaccine contains lactose as a stabilizer.
MPSV4 is administered by subcutaneous injection. The vaccine is available in single-dose and 10-dose vials. Fifty-dose vials are no longer available. Diluent for the single-dose vial is sterile water without preservative. Diluent for the 10-dose vial is sterile water with thimerosal added as a preservative. After reconstitution the vaccine is a clear colorless liquid.
Meningococcal Conjugate Vaccines
Three meningococcal conjugate vaccines are licensed in the United States: two single-component vaccines (Menactra (MenACWY-D) and Menveo (MenACWY-CRM)) and one combination vaccine with Hib (MenHibrix (Hib-MenCY-TT)).
Menactra (MenACWY-D, sanofi pasteur) was licensed in 2005. Each 0.5-mL dose of vaccine is formulated in sodium phosphate buffered isotonic sodium chloride solution to contain 4 mcg each of meningococcal A, C, W, and Y polysaccharides conjugated to approximately 48 mcg of diphtheria toxoid protein carrier. MenACWY-D is approved for use in persons 9 months through 55 years of age. It is administered by intramuscular injection. MenACWY-D is supplied as a liquid in a single-dose vial and does not contain a preservative or an adjuvant.
High-risk Groups: Functional or Anatomic Asplenia*
- Younger than 19 months
- infant series at 2, 4, 6, and 12-15 months with HibMenCY-TT or MenACWY-CRM
- 19-23 months who have not received a complete series
- 2-dose primary series of MenACWY-CRM at least 3 months apart**
- 24 months and older who have not received a complete series
- 2-dose primary series of either MenACWY at least 3 months apart**
*Including sickle-cell disease
**Doses valid if 8 weeks apart
High-risk Groups: Persistent Complement Component Deficiency
- Children 2-18 months
- Infant series at 2, 4, 6, and 12-15 months with HibMenCY-TT or MenACWY-CRM OR 2-dose primary series of MenACWY-D starting at 9 months at least 3 months apart *
- 19-23 months without complete series of HibMenCY-TT or MenACWY
- 2-dose primary series of MenACWY at least 3 months apart*
- 24 months and older who have not received a complete series of HibMenCY-TT or MenACWY
- 2-dose primary series of either MenACWY at least 3 months apart*
*Doses valid if 8 weeks apart
Menveo (MenACWY-CRM, Novartis) was licensed in the United States in 2010. MenACWY-CRM consists of two portions: 10 µg of lyophilized meningococcal serogroup A capsular polysaccharide conjugated to CRM197 (MenA) and 5 μg each of capsular polysaccharide of serogroup C, W, and Y conjugated to CRM197 in 0.5 mL of phosphate buffered saline, which is used to reconstitute the lyophilized MenA component before injection. MenACWY-CRM is approved for use in persons 2 through 55 years of age. It is administered by intramuscular injection. It does not contain a preservative or an adjuvant.
MenHibrix (Hib-MenCY-TT, GlaxoSmithKline) was licensed in the United States in 2012. Hib-MenCY-TT contains 5 micrograms of N. meningitidis serogroups C capsular polysaccharide conjugated to tetanus-toxoid, 5 micrograms of N. meningitidis serogroup Y capsular polysaccharide conjugated to tetanus-toxoid, and 2.5 micrograms of Haemophilus influenzae serogroup B capsular polysaccharide conjugated to tetanus-toxoid. The vaccine is lyophilized and should be reconstituted with a 0.9% saline diluent Hib-MenCY-TT is approved as a four dose series for children at 2, 4, 6, and 12 through 18 months.
Immunogenicity and Vaccine Efficacy
Meningococcal Polysaccharide Vaccine
The characteristics of MPSV4 are similar to other polysaccharide vaccines (e.g., pneumococcal polysaccharide). The vaccine is generally not effective in children younger than 18 months of age. The response to the vaccine is typical of a T-cell independent antigen, with an age-dependent response, and poor immunogenicity in children younger than 2 years of age. In addition, little boost in antibody titer occurs with repeated doses; the antibody which is produced is relatively low-affinity IgM, and “switching” from IgM to IgG production is poor.
The immunogenicity and clinical efficacy of serogroups A and C meningococcal polysaccharide vaccines are well-established. The serogroup A polysaccharide induces antibody response among children as young as 3 months, although a response comparable with that occurring in adults is not achieved until age 4 to 5 years; the serogroup C component is poorly immunogenic among recipients younger than 18 through 24 months. Serogroups A and C have demonstrated estimated clinical efficacies of 85% or more among school-aged children and adults during outbreaks. Although clinical protection has not been documented, vaccination with W and Y polysaccharides induces production of bactericidal antibodies. The antibody responses to each of the four polysaccharides in the quadrivalent vaccine are serogroup specific and independent (i.e., there is no cross-protection).
Meningococcal Conjugate Vaccines
Additional High-risk Groups
- Meningococcal vaccination is recommended for persons at increased risk for meningococcal disease
- microbiologists who are routinely exposed to isolates of N. meningitidis
- military recruits
- persons who travel to and U.S. citizens who reside in countries in which N. meningitides is hyperendemic or epidemic, particularly areas in the Sub-Saharan African “meningitis belt”
- Revaccinate every 5 years as long as the person remains at increased risk
Meningococcal Endemic Areas
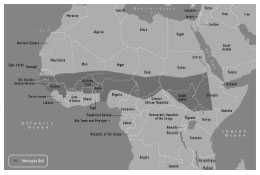
Hib-MenCY-TT and Travel
- Infants and children who received Hib-MenCY-TT and are travelling to areas with high endemic rates of meningococcal disease should receive a quadrivalent meningococcal vaccination
Effectiveness of the three meningococcal conjugate vaccines, which were licensed after MPSV4, was inferred by comparing serum bactericidal antibody assay (SBA) measurements of the new vaccine with corresponding antibody responses of the U.S.-licensed meningococcal vaccine representing the standard of care at the time (among persons aged 2 through 55 years) or by achieving a seroresponse at or above a predefined bactericidal antibody titer (among children aged 2 through 23 months).
An advantage of conjugate vaccines is their ability to elicit immunologic memory. Meningococcal conjugate vaccines prime the immune system, and immunologic memory persists even in the absence of detectible bactericidal antibodies. However, while vaccine-induced immunologic memory might be protective against infection with other disease-causing encapsulated bacteria, the presence of detectable circulating antibody appears to be important for protection against N. meningitidis. In most cases, meningococcal infection progresses rapidly, with fulminant disease occurring within 1-4 days after invasion of normally sterile body sites.
When MenACWY-D vaccine was licensed in 2005 some experts predicted that the vaccine would be effective for up to 10 years, providing protection through the period of highest risk in late adolescence and early adulthood. Since the 2005 ACIP recommendations, additional data have led to improved understanding of meningococcal conjugate vaccines, including new data on duration of vaccine-induced immunity. Antibody persistence studies indicate that circulating antibody declines 3 to 5 years after a single dose of Menactra or Menveo (MenACWY). In addition, results from a vaccine effectiveness study demonstrate waning effectiveness, and many adolescents are not protected 5 years after vaccination. ACIP concluded that a single dose of meningococcal conjugate vaccine administered at age 11 or 12 years is unlikely to protect most adolescents through the period of increased risk at ages 16 through 21 years. On the basis of this information, in 2010, ACIP recommended adding a booster dose at age 16 years.
In 2010, ACIP revised the recommendations for dosing regimens (e.g., primary series and booster doses) for persons who have functional or anatomic asplenia, who have persistent complement component deficiencies, or who have HIV infection and are otherwise recommended to be vaccinated. For these immunosuppressed persons, a 2-dose primary series was recommended instead of a single dose. Booster doses after primary vaccination are important for persons with prolonged increased risk (persons with asplenia, persons with complement component deficiencies, and microbiologists) to ensure high levels of SBA are maintained over time.
Vaccination Schedule and Use
High-risk Boosters
- Children who receive primary immunization and remain at increased risk should receive booster doses
- if primary immunization complete by 7 years of age
- first booster should be 3 years after primary immunization and every 5 years thereafter if at continued risk
- if primary immunization complete on or after 7 years of age
- first booster should be 5 years after primary immunization and every 5 years thereafter if at continued risk
- if primary immunization complete by 7 years of age
MenACWY and HIV Infection
- HIV infection is not currently an indication for MenACWY vaccination
- Some persons with HIV infection should receive MenACWY for other indications, such as adolescents or international travel
- Persons with HIV infection who are vaccinated with MenACWY should receive 2 primary series doses at least 8 weeks apart
Meningococcal Polysaccharide Vaccine
Routine vaccination of civilians with MPSV4 is not recommended. Use of MPSV4 should be limited to persons older than 55 years of age, or when neither MenACWY is available.
Meningococcal Conjugate Vaccines
ACIP recommends routine vaccination with either MenACWY vaccine at 11 or 12 years of age, with a booster dose at 16 years of age. For adolescents who receive the first dose at 13 through 15 years of age, a one-time booster dose should be administered, preferably at age 16 through 18 years. Healthy persons who receive their first routine dose of meningococcal conjugate vaccine at or after age 16 years do not need a booster dose unless they become at increased risk for meningococcal disease. Routine vaccination of healthy persons who are not at increased risk for exposure to N. meningitidis is not recommended after age 21 years. A booster dose is not recommended for healthy persons 22 years of age or older even if the first dose was administered at 11 through 15 years of age. Although doses of MenACWY separated by 8 weeks can both be counted as valid it is preferable to use a longer interval between doses, 3 to 5 years if possible.
For children younger than 19 months of age with anatomic or functional asplenia (including sickle-cell disease), administer an infant series of Hib-MenCY-TT or MenACWY-CRM at 2, 4, 6, and 12-15 months.
For children 19 through 23 months of age with anatomic or functional asplenia (including sickle-cell disease), administer two primary doses of MenACWY-CRM at least 3 months apart (doses valid If 8 weeks apart).
For children 2 through 18 months of age with persistent complement component deficiencies, administer either an infant series of Hib-MenCY-TT or MenACWY-CRM at 2, 4, 6, and 12 through 15 months or a 2-dose primary series of MenACWY-D starting at 9 months, with at least 8 weeks between doses.
For children 19 through 23 months of age with persistent complement component deficiencies who have not received a complete series of Hib-MenCY-TT or MenACWY, administer 2 primary doses of MenACWY at least 3 months apart (doses valid if 8 weeks apart).
For children 24 months of age and older with persistent complement component deficiencies or anatomic or functional asplenia (including sickle cell disease), who have not received a complete series of Hib-MenCY-TT or MenACWY, administer 2 primary doses of either MenACWY at least 3 months apart (doses valid if 8 weeks apart). Do not administer MenACWY-D to a child with asplenia (including sickle cell disease) until after the second birthday, and at least 4 weeks after completion of all PCV13 doses.
Meningococcal Vaccine Use in Outbreaks
- Both MenACWY, and MPSV4 recommended for use in control of outbreaks caused by A, C, W, and Y
- HibMenCY-TT may be used for age-appropriate persons in outbreaks specifically caused by C and Y
- Outbreak definition:
- at least 3 confirmed or probable primary cases of the same serogroup
- period of 3 months or less
- primary attack rate of more than 10 cases per 100,000 population
Meningococcal vaccination is recommended for persons at increased risk for meningococcal disease, including microbiologists who are routinely exposed to isolates of N. meningitidis, military recruits, and persons who travel to, and U.S. citizens who reside in, countries in which N. meningitidis is hyperendemic or epidemic, particularly countries in the sub-Saharan Africa “meningitis belt.” Vaccination in the 3 years before the date of travel is required by the government of Saudi Arabia for all travelers to Mecca during the annual Hajj. Information concerning geographic areas for which vaccination is recommended can be obtained from the CDC Travelers Health website . These high-risk persons should be revaccinated every 5 years as long as their increased risk continues.
Infants and children who received Hib-MenCY-TT and are travelling to areas with high endemic rates of meningococcal disease are not protected against serogroups A and W and should receive a quadrivalent meningococcal vaccination.
Children who received primary immunization with Hib-MenCY-TT, MenACWY or MPSV4 before 7 years of age and remain at increased risk for meningococcal disease should receive a booster 3 years after primary immunization. Boosters should be repeated every five years thereafter. Persons who received primary immunization with MenACWY or MPSV4 at 7 years of age or older and remain at increased risk for meningococcal disease should receive a booster 5 years after their previous dose. Boosters should be repeated every five years thereafter.
Persons with persistent complement component deficiency, and persons with functional or anatomic asplenia should receive a 2-dose primary series administered 2 months apart and a booster dose every 5 years.
HIV infection is not currently considered to be an indication for MenACWY vaccination by itself. However, some persons with HIV infection should receive MenACWY for other indications, such as adolescents or international travel. Persons with HIV infection who are vaccinated with MenACWY should receive 2 primary doses at least 8 weeks apart.
Meningococcal Vaccines Contraindications and Precautions
- Severe allergic reaction to vaccine component or following a prior dose of vaccine
- Moderate or severe acute illness
Persons with complement component deficiency, functional or anatomic asplenia or HIV infection who have already received 1 dose of MenACWY should receive a second dose at the earliest opportunity, but at least 8 weeks after the previous dose.
MenACWY can be administered at the same visit as other indicated vaccines. All vaccines should be given at separate sites with separate syringes.
Both MenACWY and MPSV4 are recommended for use in control of meningococcal outbreaks caused by vaccine-preventable serogroups (A, C, W, Y). Hib-MenCY-TT may be used for age-appropriate persons in outbreaks specifically caused by vaccine-preventable serogroups C and Y. An outbreak is defined by the occurrence of at least three confirmed or probable primary cases of the same serogroup meningococcal disease during a period of 3 months or less, with a resulting primary attack rate of 10 or more cases per 100,000 population.
MenACWY-D Adverse Events
- Fever
- most frequently reported (16.8%)
- Headache (16.0%); injection-site erythema (14.6%); dizziness (13.4%)
- Syncope
- reported in 10%
- Serious adverse events rare
- death reported in 0.3%
MenACWY-CRM Adverse Events
- Injection site swelling (13.7%)
- Injection site reactions
- most frequently reported (19.7%)
- Syncope
- reported in 8.8%
- Serious adverse events rare
- death reported in 0.4%
HibMenCY-TT Adverse Events
- Rates comparable to adverse event rates after Hib-TT
- HibMenCY-TT safe and immunogenic for both Hib and serogroups C and Y
MPSV4 Adverse Reactions
- Local reactions
- most common (48%)
- Last for one to two days
Indications for Chemoprophylaxis
- Household members
- Child care center contacts
- Anyone directly exposed to the patient’s oral secretions in 7 days before symptom onset
- Travelers with direct contact with respiratory secretions from an index patient or for anyone seated directly next to an index patient on a prolonged flight (more than 8 hours)
Contraindications and Precautions to Vaccination
Vaccination with MenACWY, MPSV4, or Hib-MenCY-TT is contraindicated for persons known to have had a severe allergic (anaphylactic) reaction to a vaccine component, including diphtheria toxoid. Recommended vaccinations can be administered to persons with minor acute illness (e.g. diarrhea or mild upper respiratory tract infection with or without low grade fever). Vaccination should be deferred for persons with moderate or severe acute illness until the condition has improved. After reviewing safety studies, ACIP voted in 2010 to remove a history Guillain-Barré syndrome (GBS) as a precaution for vaccination, because the benefits of meningococcal vaccination outweigh the risk for recurrent GBS in these persons. However, a history of GBS continues to be listed as a precaution in the package inserts for MenACWY. Breastfeeding and immunosuppression are not contraindications to vaccination. Pregnancy should not preclude vaccination with MenACWY or MPSV4, if indicated.
Adverse Events Following Vaccination
Meningococcal Conjugate Vaccine
The most frequently reported adverse events for MenACWY-D include fever (16.8%), headache (16.0%) injection site erythema (14.6%), and dizziness (13.4%). Syncope was reported in 10.0% of reports involving MenACWY-D. Of all reported MenACWY-D events, 6.6% were coded as serious (i.e., resulted in death, life-threatening illness, hospitalization, prolongation of hopitalization, or permanent disability). Serious events included headache, fever, vomiting, and nausea. A total of 24 deaths (0.3%) were reported.
The most frequently reported adverse events for MenACWY-CRM were injection site erythema (19.7%) and injection-site swelling (13.7%). Syncope was reported in 8.8% of reports involving MenACWY-CRM. One death (0.4%) was reported.
Rates of local and systemic adverse events observed after administration of Hib-MenCY-TT were comparable to rates observed after administration of Hib-TT. Thus, Hib-MenCY-TT was found to be safe and immunogenic for both Hib and meningococcal serogroups C and Y.
Meningococcal Polysaccharide Vaccine
Fever (100°F - 103°F) within 7 days of vaccination is reported for up to 3% of recipients. Systemic reactions, such as headache and malaise, within 7 days of vaccination are reported for up to 60% of recipients. Fewer than 3% of recipients reported these systemic reactions as severe.
Adverse Reactions Following Vaccination
Meningococcal Polysaccharide Vaccine
Adverse reactions to MPSV4 are generally mild. The most frequent are local reactions, such as pain and redness at the injection site. These reactions last for 1 or 2 days, and occur in up to 48% of recipients.
Vaccine Storage and Handling
MPSV4, MenACWY, and Hib-MenCY-TT should be maintained at refrigerator temperature between 35°F and 46°F (2°C and 8°C). Manufacturer’s package inserts contain additional information.For complete information on best practices and recommendations please refer to CDC’s Vaccine Storage and Handling Toolkit [4.33 MB, 109 pages].
The MenA (lyophilized) component of MenACWY-CRM should only be reconstituted using the liquid C-W-Y component of MenACWY-CRM. No other vaccine or diluents can be used for this purpose. The reconstituted vaccine should be used immediately, but may be held at or below 77°F (25°C) for up to 8 hours. If the liquid C-W-Y component of MenACWY-CRM is administered alone (without using it to reconstitute the lyophilized A component) revaccination may not be needed. Serogroup A disease is rare in the U.S. so revaccination is not necessary if the person does not plan to travel outside the U.S. However, the person should be revaccinated with either a properly reconstituted dose of MenACWY-CRM or with MenACWY-D if international travel anticipated, especially travel to Africa. There is no minimum interval between the incomplete dose given in error and the repeat dose.
Surveillance and Reporting of Meningococcal Disease
Meningococcal disease is a reportable condition in most states. Healthcare personnel should report any case of invasive meningococcal infection (meningococcal disease) to local and state health departments.
Antimicrobial Chemoprophylaxis
Timing of Chemoprophylaxis
- Should be administered as soon as possible, ideally less than 24 hours after identification of the index patient
- Chemoprophylaxis administered more than 14 days after onset of illness in the index patient probably of limited or no value
In the United States, the primary means for prevention of sporadic meningococcal disease is antimicrobial chemoprophylaxis of close contacts of infected persons. Close contacts include household members, child care center contacts, and anyone directly exposed to the patient’s oral secretions (e.g., through kissing, mouth-to-mouth resuscitation, endotracheal intubation, or endotracheal tube management) during the 7 days before symptom onset. Healthcare personnel should receive chemoprophylaxis if they were managing an airway or were exposed to respiratory secretions of a patient with meningococcal disease.
For travelers, antimicrobial chemoprophylaxis should be considered for any passenger who had direct contact with respiratory secretions from an index patient or for anyone seated directly next to an index patient on a prolonged flight (i.e., one lasting more than 8 hours). The attack rate for household contacts exposed to patients who have sporadic meningococcal disease was estimated to be four cases per 1,000 persons exposed, which is 500–800 times greater than the rate for the total population. In the United Kingdom, the attack rate among healthcare personnel exposed to patients with meningococcal disease was determined to be 25 times higher than among the general population.
Chemoprophylaxis is not recommended for close contacts of patients with evidence of Neisseria meningitidis only in nonsterile sites (e.g., oropharyngeal, endotracheal, or conjunctival). Reports of secondary cases after close contact to persons with noninvasive pneumonia or conjunctivitis are rare; there is no evidence of substantive excess risk. Furthermore, there is no indication to treat persons who are asymptomatic nasopharyngeal carriers.
Antimicrobials
- Rifampin, Ciprofloxacin, and Ceftriaxone 90%-95% effective in reducing nasopharyngeal carriage of N. meningitidis and are all acceptable for chemoprophylaxis
Because the rate of secondary disease for close contacts is highest immediately after onset of disease in the index patient, antimicrobial chemoprophylaxis should be administered as soon as possible, ideally less than 24 hours after identification of the index patient. Conversely, chemoprophylaxis administered more than 14 days after onset of illness in the index patient is probably of limited or no value. Oropharyngeal or nasopharyngeal cultures are not helpful in determining the need for chemoprophylaxis and might unnecessarily delay institution of this preventive measure.
Rifampin, ciprofloxacin, and ceftriaxone are 90%–95% effective in reducing nasopharyngeal carriage of N. meningitidis and are all acceptable antimicrobial agents for chemoprophylaxis. Although sporadic resistance to rifampin and ciprofloxacin has been reported worldwide, meningococcal resistance to chemoprophylaxis antibiotics remains rare in the United States. Clinicians should report suspected chemoprophylaxis failures to their public health departments. Systemic antimicrobial therapy for meningococcal disease with agents other than ceftriaxone or other third-generation cephalosporins might not reliably eradicate nasopharyngeal carriage of N. meningitidis. If other agents have been used for treatment, the index patient should receive chemoprophylactic antibiotics for eradication of nasopharyngeal carriage before being discharged from the hospital.
Acknowledgement
The editors thank Drs. Amanda Cohn and Gina Mootrey, CDC for their assistance in updating this chapter.
Selected References
- CDC. Active Bacterial Core surveillance (ABCs).
- CDC. Manual for the Prevention of Vaccine-Preventable Diseases. 5th edition, 2011.
- CDC. Prevention and Control of Meningococcal Disease: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2013;62(No. RR-2):[1-30].
- CDC. Updated recommendation from the Advisory Committee on Immunization Practices (ACIP) for revaccination of persons at prolonged increased risk for meningococcal disease. MMWR 2009;58(No.37):1042-3.
- CDC. Updated recommendations for use of meningococcal conjugate vaccines -Advisory Committee on Immunization Practices (ACIP). MMWR 2011;60(No.3):72-6.
- CDC. Recommendations of the Advisory Committee on Immunization Practices (ACIP) for use of quadrivalent meningococcal conjugate vaccine (MenACWY-D) among children aged 9 through 23 months at increased risk for invasive meningococcal disease. MMWR 2011;60(No.40):1391-2.
- Granoff DM, Harrison L, Borrow R. Meningococcal vaccine. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. 5th ed. China: Saunders; 2008: 399–434.
- Harrison LH, Pass MA, Mendelsohn AB, et al. Invasive meningococcal disease in adolescents and young adults. JAMA 2001;286:694–9.
- Jodar L, Feavers IM, Salisbury D, Granoff DM. Development of vaccines against meningococcal disease. Lancet 2002;359(9316):1499–1508.
- Rosenstein NE, Perkins BA, Stephens DS, et al. Meningococcal disease. N Engl J Med 2001;344:1378–88.
- Shepard CW, Ortega-Sanchez IR, Scott RD, Rosenstein NE; ABCs Team. Cost-effectiveness of conjugate meningococcal vaccination strategies in the United States. Pediatrics 2005;115:1220–32.
- Sejvar JJ, Johnson D, Popovic T, et al. Assessing the risk of laboratory-acquired meningococcal disease. J Clin Microbiolol 2005;43:4811–4.
- Page last reviewed: November 15, 2016
- Page last updated: July 24, 2015
- Content source:


 ShareCompartir
ShareCompartir