Rubella
On this Page
Rubella
- From Latin meaning "little red"
- Discovered in 18th century - thought to be variant of measles
- First described as distinct clinical entity in German literature
- Congenital rubella syndrome (CRS) described by Gregg in 1941
- Rubella virus first isolated in 1962 by Parkman and Weller
Rubella Virus
- Togavirus
- RNA virus
- One antigenic type
- Inactivated by lipid solvents, trypsin, formalin, ultraviolet light, low pH, heat, and amantadine
Rubella Pathogenesis
- Respiratory transmission of virus
- Replication in nasopharynx and regional lymph nodes
- Viremia 5 to 7 days after exposure with spread throughout body
- Transplacental infection of fetus during viremia
The name rubella is derived from Latin, meaning “little red.” Rubella was initially considered to be a variant of measles or scarlet fever and was called “third disease”. It was not until 1814 that it was first described as a separate disease in the German medical literature, hence the common name “German measles”. In 1914, Hess postulated a viral etiology based on his work with monkeys. Hiro and Tosaka in 1938 confirmed the viral etiology by passing the disease to children using filtered nasal washings from persons with acute cases.
Following a widespread epidemic of rubella infection in 1940, Norman Gregg, an Australian ophthalmologist, reported in 1941 the occurrence of congenital cataracts among 78 infants born following maternal rubella infection in early pregnancy. This was the first published recognition of congenital rubella syndrome (CRS). Rubella virus was first isolated in 1962 by Parkman and Weller. The first rubella vaccines were licensed in 1969.
Rubella Virus
Rubella virus is classified as a togavirus, genus Rubivirus. It is most closely related to group A arboviruses, such as eastern and western equine encephalitis viruses. It is an enveloped RNA virus, with a single antigenic type that does not cross-react with other members of the togavirus group. Rubella virus is relatively unstable and is inactivated by lipid solvents, trypsin, formalin, ultraviolet light, low pH, heat, and amantadine.
Pathogenesis
Following respiratory transmission of rubella virus, replication of the virus is thought to occur in the nasopharynx and regional lymph nodes. A viremia occurs 5 to 7 days after exposure with spread of the virus throughout the body. Transplacental infection of the fetus occurs during viremia. Fetal damage occurs through destruction of cells as well as mitotic arrest.
Clinical Features
Acquired Rubella
Rubella Clinical Features
- Incubation period 14 days (range 12-23 days)
- Prodrome is rare in children
- Prodrome of low-grade fever in adults
- Maculopapular rash 14 to 17 days after exposure
- Lymphadenopathy occurs before rash and lasts for several weeks
Rubella Complications
- Arthralgia or arthritis (adult female) - up to 70%
- Arthralgia or arthritis (children) - rare
- Encephalitis - 1/6,000 cases
- Hemorraghic manifestations (e.g. thrombocytopenic purpura) - 1/3000
- Orchitis, neuritis, progressive panencephalitis - rare
Epidemic Rubella - United States, 1964-1965
- 12.5 million rubella cases
- 20,000 CRS cases
- Estimated cost more than $840 million
The incubation period of rubella is 14 days, with a range of 12 to 23 days. Symptoms are often mild, and up to 50% of infections may be subclinical or inapparent. In children, rash is usually the first manifestation and a prodrome is rare. In older children and adults, there is often a 1 to 5 day prodrome with low-grade fever, malaise, lymphadenopathy, and upper respiratory symptoms preceding the rash. The rash of rubella is maculopapular and occurs 14 to 17 days after exposure. The rash usually occurs initially on the face and then progresses from head to foot. It lasts about 3 days and is occasionally pruritic. The rash is fainter than measles rash and does not coalesce. The rash is often more prominent after a hot shower or bath. Lymphadenopathy may begin a week before the rash and last several weeks. Postauricular, posterior cervical, and suboccipital nodes are commonly involved.
Arthralgia and arthritis occur so frequently in adults that they are considered by many to be an integral part of the illness rather than a complication. Other symptoms of rubella include conjunctivitis, testalgia, or orchitis. Forschheimer spots may be noted on the soft palate but are not diagnostic for rubella.
Complications
Complications of rubella are not common, but they generally occur more often in adults than in children.
Arthralgia or arthritis may occur in up to 70% of adult women who contract rubella, but it is rare in children and adult males. Fingers, wrists, and knees are often affected. Joint symptoms tend to occur about the same time or shortly after appearance of the rash and may last for up to 1 month; chronic arthritis is rare.
Encephalitis occurs in one in 6,000 cases, more frequently in adults (especially in females) than in children. Mortality estimates vary from 0 to 50%.
Hemorrhagic manifestations occur in approximately one per 3,000 cases, occurring more often in children than in adults. These manifestations may be secondary to low platelets and vascular damage, with thrombocytopenic purpura being the most common manifestation. Gastrointestinal, cerebral, or intrarenal hemorrhage may occur. Effects may last from days to months, and most patients recover.
Additional complications include orchitis, neuritis, and a rare late syndrome of progressive panencephalitis.
Congenital Rubella Syndrome
Prevention of CRS is the main objective of rubella vaccination programs in the United States.
A rubella epidemic in the United States in 1964–1965 resulted in 12.5 million cases of rubella infection and about 20,000 newborns with CRS. The estimated cost of the epidemic was $840 million. This does not include the emotional toll on the families involved.
Infection with rubella virus is most severe in early gestation. The virus may affect all organs and cause a variety of congenital defects. Infection may lead to fetal death, spontaneous abortion, or preterm delivery. The severity of the effects of rubella virus on the fetus depends largely on the time of gestation at which infection occurs. As many as 85% of infants infected in the first trimester of pregnancy will be found to be affected if followed after birth. While fetal infection may occur throughout pregnancy, defects are rare when infection occurs after the 20th week of gestation. The overall risk of defects during the third trimester is probably no greater than that associated with uncomplicated pregnancies.
Congenital Rubella Syndrome
- Infection may affect all organs
- May lead to fetal death or premature delivery
- Severity of damage to fetus depends on gestational age
- Up to 85% of infants affected if infected during first trimester
- Deafness
- Eye defects
- Cardiac defects
- Microcephaly
- Mental retardation
- Bone alterations
- Liver and spleen damage
Rubella Laboratory Diagnosis
- Isolation of rubella virus from clinical specimen (e.g., nasopharynx, urine)
- Serologic tests available vary among laboratories
- Positive serologic test for rubella IgM antibody
- Significant rise in rubella IgG by any standard serologic assay (e.g., enzyme immunoassay)
Congenital infection with rubella virus can affect virtually all organ systems. Deafness is the most common and often the sole manifestation of congenital rubella infection, especially after the fourth month of gestation. Eye defects, including cataracts, glaucoma, retinopathy, and microphthalmia may occur. Cardiac defects such as patent ductus arteriosus, ventricular septal defect, pulmonic stenosis, and coarctation of the aorta are possible. Neurologic abnormalities, including microcephaly and mental retardation, and other abnormalities, including bone lesions, splenomegaly, hepatitis, and thrombocytopenia with purpura may occur.
Manifestations of CRS may be delayed from 2 to 4 years. Diabetes mellitus appearing in later childhood occurs frequently in children with CRS. In addition, progressive encephalopathy resembling subacute sclerosing panencephalitis has been observed in some older children with CRS. Children with CRS have a higher than expected incidence of autism.
Infants with CRS may have low titers by hemagglutination inhibition (HI) but may have high titers of neutralizing antibody that may persist for years. Reinfection may occur. Impaired cell-mediated immunity has been demonstrated in some children with CRS.
Laboratory Diagnosis
Many rash illnesses can mimic rubella infection, and as many as 50% of rubella infections may be subclinical. The only reliable evidence of acute rubella infection is a positive viral culture for rubella or detection of rubella virus by polymerase chain reaction (PCR), the presence of rubella-specific IgM antibody, or demonstration of a significant rise in IgG antibody from paired acute- and convalescent-phase sera.
Rubella virus can be isolated from nasal, blood, throat, urine and cerebrospinal fluid specimens from rubella and CRS patients. Virus may be isolated from the pharynx 1 week before and until 2 weeks after rash onset. Although isolation of the virus is diagnostic of rubella infection, viral cultures are labor intensive, and therefore not done in many laboratories; they are generally not used for routine diagnosis of rubella. Viral isolation is an extremely valuable epidemiologic tool and should be attempted for all suspected cases of rubella or CRS. Information about rubella virus isolation can be found on the CDC website.
Serology is the most common method of confirming the diagnosis of rubella. Acute rubella infection can be serologically confirmed by a significant rise in rubella antibody titer in acute- and convalescent-phase serum specimens or by the presence of serum rubella IgM. Serum should be collected as early as possible (within 7–10 days) after onset of illness, and again 14–21 days (minimum of 7) days later.
False-positive serum rubella IgM tests have occurred in persons with parvovirus infections, with a positive heterophile test for infectious mononucleosis, or with a positive rheumatoid factor.
The serologic tests available for laboratory confirmation of rubella infections vary among laboratories. The state health department can provide guidance on available laboratory services and preferred tests.
Enzyme-linked immunosorbent assay (ELISA) is sensitive, widely available, and relatively easy to perform. It can also be modified to measure IgM antibodies. Most of the diagnostic testing done for rubella antibodies uses some variation of ELISA.
Epidemiology
Rubella Epidemiology
- Reservoir
- human
- Transmission
- respiratory (Subclinical cases may transmit)
- Temporal pattern
- peak in late winter and spring
- Communicability
- 7 days before to 5-7 days after rash onset
- Infants with CRS may shed virus for a year or more
Occurrence
Rubella occurs worldwide. See information about clinical case definition, clinical classification and epidemiologic classification of rubella and congenital rubella syndrome.
Reservoir
Rubella is a human disease. There is no known animal reservoir. Although infants with CRS may shed rubella virus for an extended period, a true carrier state has not been described.
Transmission
Rubella is spread from person to person via airborne transmission or droplets shed from the respiratory secretions of infected persons. There is no evidence of insect transmission. Rubella may be transmitted by persons with subclinical or asymptomatic cases (up to 50% of all rubella virus infections).
Temporal Pattern
In temperate areas, incidence is usually highest in late winter and early spring.
Communicability
Rubella is only moderately contagious. The disease is most contagious when the rash first appears, but virus may be shed from 7 days before to 5–7 days or more after rash onset.
Infants with CRS shed large quantities of virus from body secretions for up to 1 year and can therefore transmit rubella to persons caring for them who are susceptible to the disease.
Secular Trends in the United States
Rubella - United States, 1966-2011
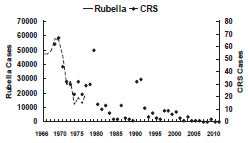
Rubella and congenital rubella syndrome became nationally notifiable diseases in 1966. The largest annual total of cases of rubella in the United States was in 1969, when 57,686 cases were reported (58 cases per 100,000 population). Following vaccine licensure in 1969, rubella incidence declined rapidly. By 1983, fewer than 1,000 cases per year were reported (less than 0.5 cases per 100,000 population). A moderate resurgence of rubella occurred in 1990–1991, primarily due to outbreaks in California (1990) and among the Amish in Pennsylvania (1991). In 2003, a record low annual total of seven cases was reported. In October 2004, CDC convened an independent expert panel to review available rubella and CRS data. After a careful review, the panel unanimously agreed that rubella was no longer endemic in the United States. The number of reported cases of rubella in the United States remains low with a median of 11 cases annually in 2005-2011.
Rubella - United States, 1980-2011
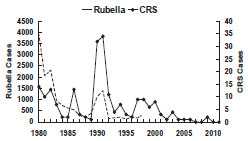
Until recently, there was no predominant age group for rubella cases. During 1982 through 1992, approximately 30% of cases occurred in children younger than 5 years, 30% occurred in children 5 through 14 years, and 30% occurred in persons 15 through 39 years. Adults 40 years of age and older typically accounted for less than 10% of cases. Since 2004 when endemic rubella was declared eliminated in the U.S., persons 20-49 years of age have accounted for 60 percent of the cases (median age 32 years).
Most reported rubella in the United States in the mid-1990s has occurred among Hispanic young adults who were born in areas where rubella vaccine is routinely not given. In 1998, Latin America nations and Mexico began major rubella control efforts, which resulted in a marked decrease in the number of rubella cases.
Rubella and CRS in the United States
- Most reported rubella in the U.S. in the mid-1990s has occurred among foreign-born Hispanic young adults
- Indigenous transmission of rubella determined to have ended in 2004
- In 2010 PAHO announced region of the Americas achieved rubella and CRS elimination goal
CRS surveillance is maintained through the National Congenital Rubella Registry, which is managed by the National Center for Immunization and Respiratory Diseases. The largest annual total of reported CRS cases to the registry was in 1970 (67 cases). An average of 2-3 CRS cases were reported annually during 1998-2012. Although reported rubella activity has consistently and significantly decreased since vaccine has been used in the United States, the incidence of CRS has paralleled the decrease in rubella cases only since the mid-1970s. The decline in CRS since the mid-1970s was due to an increased effort to vaccinate susceptible adolescents and young adults, especially women. Rubella outbreaks are almost always followed by an increase in CRS.
Rubella outbreaks in California and Pennsylvania in 1990–1991 resulted in 25 cases of CRS in 1990 and 33 cases in 1991. From 2004-2012, a total of 6 CRS cases were reported in the U.S., 5 of which where the mother was likely infected while in Asia or Africa. In 2010, the Pan American Health Organization (PAHO) announced that the Region of the Americas had achieved the rubella and CRS elimination goals set in 2003 based on surveillance data. Although regional documentation of elimination is ongoing, an expert panel unanimously agreed in December 2011 that rubella elimination has been maintained in the United States.
Rubella Vaccine
Three rubella vaccines were licensed in the United States in 1969: HPV-77:DE-5 Meruvax (duck embryo), HPV-77:DK-12 Rubelogen (dog kidney), and GMK-3:RK53 Cendevax (rabbit kidney) strains. HPV-77:DK-12 was later removed from the market because there was a higher rate of joint complaints following vaccination with this strain. In 1979, the RA 27/3 (human diploid fibroblast) strain (Meruvax-II, Merck) was licensed and all other strains were discontinued.
Rubella Vaccine
| Vaccine | Trade Name | Licensure | Discontinued |
|---|---|---|---|
| HPV-77:DE5 | Meruvax | 1969 | 1979 |
| HPV-77:DK12 | Rubelogen | 1969 | 1979 |
| GMK-3:RK53 | Cendevax | 1969 | 1979 |
| RA 27/3* | Meruvax II | 1979 | Still in use |
*Only vaccine currently licensed in U.S.
Characteristics
Rubella Vaccine
- Composition
- live virus (RA 27/3 strain)
- Efficacy
- 95% or more
- Duration of Immunity
- lifelong
- Schedule
- at least 1 dose
- Should be administered with measles and mumps as MMR or with measles, mumps and varicella as MMRV
The RA 27/3 rubella vaccine is a live attenuated virus. It was first isolated in 1965 at the Wistar Institute from a rubella-infected aborted fetus. The virus was attenuated by 25–30 passages in tissue culture, using human diploid fibroblasts. It does not contain duck, chicken or egg protein.
Vaccine virus is not communicable except in the setting of breastfeeding (see Contraindications Section), even though virus may be cultured from the nasopharynx of vaccinees.
Rubella vaccine is available combined with measles and mumps vaccines as MMR, or combined with mumps, measles, and varicella vaccine as MMRV (ProQuad). The Advisory Committee on Immunization Practices (ACIP) recommends that combined measles-mumps-rubella vaccine (MMR) be used when any of the individual components is indicated. Single-antigen rubella vaccine is not available in the U.S.
MMR and MMRV are supplied as a lyophylized (freeze-dried) powder and are reconstituted with sterile, preservative-free water. The vaccines contains a small amount of human albumin, neomycin, sorbitol, and gelatin.
Immunogenicity and Vaccine Efficacy
RA 27/3 rubella vaccine is safe and more immunogenic than rubella vaccines used previously. In clinical trials, 95% or more of vaccinees aged 12 months and older developed serologic evidence of rubella immunity after a single dose. More than 90% of vaccinated persons have protection against both clinical rubella and viremia for at least 15 years. Follow-up studies indicate that one dose of vaccine confers long-term, probably lifelong, protection. Seroconversion rates are similar for single-antigen rubella vaccine, MMR, and MMRV.
Several reports indicate that viremic reinfection following exposure may occur in vaccinated persons who have low levels of detectable antibody. The frequency and consequences of this phenomenon are unknown, but it is believed to be uncommon. Rarely, clinical reinfection and fetal infection have been reported among women with vaccine-induced immunity. Rare cases of CRS have occurred among infants born to women who had documented serologic evidence of rubella immunity before they became pregnant.
Vaccination Schedule and Use
At least one dose of rubella-containing vaccine, as combination MMR (or MMRV) vaccine, is routinely recommended for all children 12 months of age or older. MMRV is approved for ages 12 months through 12 years (that is, until the 13th birthday) and should not be adminstered to persons 13 years or older. All persons born during or after 1957 should have documentation of at least one dose of MMR. The first dose of MMR should be given on or after the first birthday. Any dose of rubella-containing vaccine given before 12 months of age should not be counted as part of the series. Children vaccinated with rubella-containing vaccine before 12 months of age should be revaccinated when the child is at least 12 months of age.
Rubella Vaccine (MMR) Indications
- All infants 12 months of age and older
- Susceptible adolescents and adults without documented evidence of rubella immunity
- Emphasis on nonpregnant women of childbearing age, particularly those born outside the U.S.
- Emphasis on males and females in college, places of employment, and health care settings
A second dose of MMR is recommended to produce immunity to measles and mumps in those who failed to respond to the first dose. Data indicate that almost all persons who do not respond to the measles component of the first dose will respond to a second dose of MMR. Few data on the immune response to the rubella and mumps components of a second dose of MMR are available. However, most persons who do not respond to the rubella or mumps component of the first MMR dose would be expected to respond to the second dose. The second dose is not generally considered a booster dose because a primary immune response to the first dose provides long-term protection. Although a second dose of vaccine may increase antibody titers in some persons who responded to the first dose, available data indicate that these increased antibody titers are not sustained. The combined MMR vaccine is recommended for both doses to ensure immunity to all three viruses.
The second dose of MMR vaccine should routinely be given at age 4 through 6 years, before a child enters kindergarten or first grade. The recommended health visit at age 11 or 12 years can serve as a catch-up opportunity to verify vaccination status and administer MMR vaccine to those children who have not yet received two doses of MMR (with the first dose administered no earlier than the first birthday). The second dose of MMR may be administered as soon as 1 month (i.e., minimum of 28 days) after the first dose. The minimum interval between doses of MMRV is 3 months.
All older children not previously immunized should receive at least one dose of rubella vaccine as MMR or MMRV if 12 years of age or younger.
Adults born in 1957 or later who do not have a medical contraindication should receive at least one dose of MMR vaccine unless they have documentation of vaccination with at least one dose of measles-, mumps-, and rubella-containing vaccine or other acceptable evidence of immunity to these three diseases. Some adults at high risk of measles and mumps exposure may require a second dose. This second dose should be administered as combined MMR vaccine (see Measles chapter for details). Efforts should be made to identify and vaccinate susceptible adolescents and adults, particularly women of childbearing age who are not pregnant. Particular emphasis should be placed on vaccinating both males and females in colleges, places of employment, and healthcare settings.
Only doses of vaccine with written documentation of the date of receipt should be accepted as valid. Self-reported doses or a parental report of vaccination is not considered adequate documentation. A healthcare provider should not provide an immunization record for a patient unless that healthcare provider has administered the vaccine or has seen a record that documents vaccination. Persons who lack adequate documentation of vaccination or other acceptable evidence of immunity should be vaccinated. Vaccination status and receipt of all vaccinations should be documented in the patient’s permanent medical record and in a vaccination record held by the individual.
For the first dose of measles, mumps, rubella, and varicella vaccines at age 12 through 47 months, either MMR vaccine and varicella vaccine or MMRV vaccine may be used. Providers who are considering administering MMRV vaccine should discuss the benefits and risks of both vaccination options with the parents or caregivers. Unless the parent or caregiver expresses a preference for MMRV vaccine, CDC recommends that MMR vaccine and varicella vaccine should be administered for the first dose in this age group. For the second dose of measles, mumps, rubella, and varicella vaccines at any age (15 months through 12 years) and for the first dose at 48 months of age or older, use of MMRV vaccine generally is preferred over separate injections of its equivalent component vaccines (i.e., MMR vaccine and varicella vaccine).
Rubella Immunity
Rubella Immunity
- Documentation of one dose of rubella-containing vaccine on or after the first birthday
- Serologic evidence of immunity
- Birth before 1957 (except women of childbearing age)
- Birth before 1957 is not acceptable evidence of rubella immunity for women who might become pregnant
- Only serology or documented vaccination should be accepted
Persons generally can be considered immune to rubella if they have documentation of vaccination with at least one dose of MMR (or MMRV) or other live rubella-containing vaccine administered on or after their first birthday, have serologic evidence of rubella immunity, or were born before 1957. Persons who have an “equivocal” serologic test result should be considered rubella-susceptible. Although only one dose of rubella-containing vaccine is required as acceptable evidence of immunity to rubella, children should receive two doses of MMR vaccine according to the routine childhood vaccination schedule.
Birth before 1957 provides only presumptive evidence of rubella immunity; it does not guarantee that a person is immune to rubella. Because rubella can occur in some unvaccinated persons born before 1957 and because congenital rubella and congenital rubella syndrome can occur in the offspring of women infected with rubella during pregnancy, birth before 1957 is not acceptable evidence of rubella immunity for women who might become pregnant. Only a positive serologic test for rubella antibody or documentation of appropriate vaccination should be accepted for women who may become pregnant
Healthcare personnel born before 1957 also should not be presumed to be immune. Medical facilities should consider recommending at least one dose of MMR vaccine to unvaccinated healthcare personnel born before 1957 who do not have laboratory evidence of rubella immunity. Rubella vaccination or laboratory evidence of rubella immunity is particularly important for healthcare personnel who could become pregnant, including those born before 1957. This recommendation is based on serologic studies which indicate that among hospital personnel born before 1957, 5% to 9% had no detectable measles antibody.
MMR Vaccine Contraindications and Precautions
- History of anaphylactic reactions to neomycin
- History of severe allergic reaction to any component of the vaccine
- Pregnancy
- Immunosuppression
- Moderate or severe acute illness
- Recent blood product
- Personal or family (i.e., sibling or parent) history of seizures of any etiology (MMRV only)
Clinical diagnosis of rubella is unreliable and should not be considered in assessing immune status. Because many rash illnesses may mimic rubella infection and many rubella infections are unrecognized, the only reliable evidence of previous rubella infection is the presence of serum rubella IgG antibody. Laboratories that regularly perform antibody testing are generally the most reliable because their reagents and procedures are strictly standardized.
Serologic screening need not be done before vaccinating for measles and rubella unless the medical facility considers it cost-effective. Serologic testing is appropriate only if tracking systems are used to ensure that tested persons who are identified as susceptible are subsequently vaccinated in a timely manner. If the return and timely vaccination of those screened cannot be assured, vaccination should be done without prior testing. Serologic testing for immunity to measles and rubella is not necessary for persons documented to be appropriately vaccinated or who have other acceptable evidence of immunity.
Neither rubella vaccine nor immune globulin is effective for postexposure prophylaxis of rubella. Vaccination after exposure is not harmful and may possibly avert later disease.
Contraindications and Precautions to Vaccination
Contraindications for MMR and MMRV vaccines include history of anaphylactic reactions to neomycin, history of severe allergic reaction to any component of the vaccine, and immunosuppression. Women known to be pregnant or attempting to become pregnant should not receive rubella vaccine. Although there is no evidence that rubella vaccine virus causes fetal damage, pregnancy should be avoided for 4 weeks (28 days) after rubella or MMR vaccination.
Persons with immunodeficiency or immunosuppression, resulting from leukemia, lymphoma, generalized malignancy, immune deficiency disease, or immunosuppressive therapy should not be vaccinated. However, treatment with low-dose (less than 2 mg/kg/day), alternate-day, topical, or aerosolized steroid preparations is not a contraindication to rubella vaccination. Persons whose immunosuppressive therapy with steroids has been discontinued for 1 month (3 months for chemotherapy) may be vaccinated. Rubella vaccine should be considered for persons with asymptomatic or mildly symptomatic HIV infection. See Measles chapter for additional details on vaccination of immunosuppressed persons, including those with human immunodeficiency virus infection.
Persons with moderate or severe acute illness should not be vaccinated until the illness has improved. Minor illness (e.g., otitis media, mild upper respiratory infections), concurrent antibiotic therapy, and exposure or recovery from other illnesses are not contraindications to rubella vaccination.
Receipt of antibody-containing blood products (e.g., immune globulin, whole blood or packed red blood cells, intravenous immune globulin) may interfere with seroconversion to rubella vaccine. Vaccine should be given 2 weeks before, or deferred for at least 3 months following administration of an antibody-containing blood product. If rubella vaccine is given as combined MMR, a longer delay may be necessary before vaccination. For more information, see Chapter 2, General Recommendations on Immunization.
Previous administration of human anti-Rho(D) immune globulin (RhoGam) does not generally interfere with an immune response to rubella vaccine and is not a contraindication to postpartum vaccination. However, women who have received anti-Rho immune globulin should be serologically tested 6–8 weeks after vaccination to ensure that seroconversion has occurred.
A personal or family (i.e., sibling or parent) history of seizures of any etiology is a precaution for MMRV vaccination. Studies suggest that children who have a personal or family history of febrile seizures or family history of epilepsy are at increased risk for febrile seizures compared with children without such histories. Children with a personal or family history of seizures of any etiology generally should be vaccinated with MMR vaccine and varicella vaccine (for the first dose) because the risks for using MMRV vaccine in this group of children generally outweigh the benefits.
Although vaccine virus may be isolated from the pharynx, vaccinees do not transmit rubella to others, except occasionally in the case of the vaccinated breastfeeding woman. In this situation, the infant may be infected, presumably through breast milk, and may develop a mild rash illness, but serious effects have not been reported. Infants infected through breastfeeding have been shown to respond normally to rubella vaccination at 12–15 months of age. Breastfeeding is not a contraindication to rubella vaccination and does not alter rubella vaccination recommendations.
Adverse Events Following Vaccination
MMR Adverse Events
- Fever
- Rash
- Chronic arthralgias
- Chronic arthritis
- Transient peripheral neuritic complaints
- Recurrent joint symptoms
- Collagen disease
MMR Adverse Reactions
- Fever
- Lymphadenopathy
- Arthralgia – associated with rubella component
- Arthritis- associated with rubella component
- Pain, paresthesia – begins 1-3 weeks after vaccination, persist for 1 day to three weeks, and rarely recurs
Rubella Vaccine Arthropathy
- Acute arthralgia in about 25% of vaccinated, susceptible adult women
- Acute arthritis-like signs and symptoms occurs in about 10% of recipients
- Rare reports of chronic or persistent symptoms
Rubella vaccine is very safe. Most adverse events reported following MMR vaccination (such as fever and rash) are attributable to the measles component. Data from studies in the United States and experience from other countries using the RA 27/3 strain rubella vaccine have not supported an association between the vaccine and chronic arthritis. The Institute of Medicine found that evidence was inadequate to accept or reject a causal relationship between MMR vaccine and chronic arthralgia or arthritis in women. Rarely, transient peripheral neuritic complaints, such as paresthesias and pain in the arms and legs, have been reported. One study among 958 seronegative immunized and 932 seronegative unimmunized women aged 15–39 years found no association between rubella vaccination and development of recurrent joint symptoms, neuropathy, or collagen disease.
Adverse Reactions Following Vaccination
The most common complaints following rubella vaccination are fever, lymphadenopathy, and arthralgia. These reactions only occur in susceptible persons and are more common in adults, especially in women.
Joint symptoms, such as arthralgia (joint pain) and arthritis (joint redness and/or swelling), are associated with the rubella component of MMR. Arthralgia and transient arthritis occur more frequently in susceptible adults than in children and more frequently in susceptible women than in men. Acute arthralgia or arthritis is rare following vaccination of children with RA 27/3 vaccine. By contrast, approximately 25% of susceptible postpubertal females develop acute arthralgia following RA 27/3 vaccination, and approximately 10% have been reported to have acute arthritis-like signs and symptoms.
When acute joint symptoms occur, or when pain or paresthesias not associated with joints occur, the symptoms generally begin 1–3 weeks after vaccination, persist for 1 day to 3 weeks, and rarely recur. Adults with acute joint symptoms following rubella vaccination rarely have had to disrupt work activities.
The ACIP continues to recommend the vaccination of all adult women who do not have evidence of rubella immunity.
See the Measles and Varicella chapters for information about adverse reactions following MMRV vaccine.
Rubella Vaccination of Women of Childbearing Age
Vaccination of Woman of Childbearing Age
- Ask if pregnant or likely to become so in next 4 weeks
- Exclude those who say "yes"
- For others
- explain theoretical risks
- vaccinate
Vaccination in Pregnancy Study 1971-1989
- 321 women vaccinated
- 324 live births
- No observed CRS
- Maximum theoretical risk of 1.6%, based on confidence limits (1.2% for all types of rubella vaccine)
Women who are pregnant or who intend to become pregnant within 4 weeks should not receive rubella vaccine. ACIP recommends that vaccine providers ask a woman if she is pregnant or likely to become pregnant in the next 4 weeks. Those who are pregnant or intend to become pregnant should not be vaccinated. All other women should be vaccinated after being informed of the theoretical risks of vaccination during pregnancy and the importance of not becoming pregnant during the 4 weeks following vaccination. ACIP does not recommend routine pregnancy screening of women before rubella vaccination.
If a pregnant woman is inadvertently vaccinated or if she becomes pregnant within 4 weeks after vaccination, she should be counseled about the concern for the fetus (see below), but MMR vaccination during pregnancy should not ordinarily be a reason to consider termination of the pregnancy.
When rubella vaccine was licensed, concern existed about women being inadvertently vaccinated while they were pregnant or shortly before conception. This concern came from the known teratogenicity of the wild-virus strain. To determine whether CRS would occur in infants of such mothers, CDC maintained a registry from 1971 to 1989 of women vaccinated during pregnancy. This was called the Vaccine in Pregnancy (VIP) Registry.
Although subclinical fetal infection has been detected serologically in approximately 1%–2% of infants born to susceptible vaccinees, regardless of the vaccine strain, the data collected by CDC in the VIP Registry showed no evidence of CRS occurring in offspring of the 321 susceptible women who received rubella vaccine and who continued pregnancy to term. The observed risk of vaccine-induced malformation was 0%, with a maximum theoretical risk of 1.6%, based on 95% confidence limits (1.2% for all types of rubella vaccine). Since the risk of the vaccine to the fetus appears to be extremely low, if it exists at all, routine termination of pregnancy is not recommended. Individual counseling for these women is recommended. As of April 30, 1989, CDC discontinued the VIP registry.
The ACIP continues to state that because of the small theoretical risk to the fetus of a vaccinated woman, pregnant women should not be vaccinated.
Vaccine Storage and Handling
MMR vaccine can be stored either in the freezer or the refrigerator and should be protected from light at all times. MMRV vaccine should be stored frozen between -58°F and +5°F (-50°C to -15°C). When MMR vaccine is stored in the freezer, the temperature should be the same as that required for MMRV, between -58°F and +5°F (-50°C to -15°C). Storing MMR in the freezer with MMRV may help prevent inadvertent storage of MMRV in the refrigerator.
Manufacturer package inserts contain additional information. For complete information on best practices and recommendations please refer to CDC’s Vaccine Storage and Handling Toolkit [4.33 MB, 109 pages].
Strategies to Decrease Rubella and CRS
Vaccination of Susceptible Postpubertal Females
Elimination of indigenous rubella and CRS can be maintained by continuing efforts to vaccinate susceptible adolescents and young adults of childbearing age, particularly those born outside the United States. These efforts should include vaccinating in family planning clinics, sexually transmitted disease (STD) clinics, and as part of routine gynecologic care; maximizing use of premarital serology results; emphasizing immunization for college students; vaccinating women postpartum and postabortion; immunizing prison staff and, when possible, prison inmates, especially women inmates; offering vaccination to at-risk women through the special supplemental program for Women, Infants and Children (WIC); and implementing vaccination programs in the workplace, particularly those employing persons born outside the United States.
Hospital Rubella Programs
Emphasis should be placed on vaccinating susceptible hospital personnel, both male and female (e.g., volunteers, trainees, nurses, physicians.) Ideally, all hospital employees should be immune. It is important to note that screening programs alone are not adequate. Vaccination of susceptible staff must follow.
Top of PageAcknowledgement
The editors thank Drs. Greg Wallace, and Zaney Leroy, CDC for their assistance in updating this chapter.
Selected References
- American Academy of Pediatrics. Rubella. In: Pickering L, Baker C, Kimberlin D, Long S, eds. Red Book: 2009 Report of the Committee on Infectious Diseases. 28th ed. Elk Grove Village, IL: American Academy of Pediatrics, 2009:579–84.
- CDC. Measles, mumps, and rubella—vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 1998;47(No. RR-8):1–57.
- CDC. Immunization of health-care personnel. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2011;60(RR-7):1-45.
- CDC. Control and prevention of rubella: evaluation and management of suspected outbreaks, rubella in pregnant women, and surveillance for congenital rubella syndrome. MMWR 2001;50(No. RR-12):1–30.
- CDC. Rubella vaccination during pregnancy—United States,1971–1988. MMWR 1989;38:289–93.
- CDC. Notice to readers. Revised ACIP recommendations for avoiding pregnancy after receiving rubella-containing vaccine. MMWR 2001;50:1117.
- CDC. Use of combination measles, mumps, rubella, and varicella vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2010;59(No. RR-3):1–12.
- Frenkel LM, Nielsen K, Garakian A, et al. A search for persistent rubella virus infection in persons with chronic symptoms after rubella and rubella immunization and in patients with juvenile rheumatoid arthritis. Clin Infect Dis 1996;22:287–94.
- Mellinger AK, Cragan JD, Atkinson WL, et al. High incidence of congenital rubella syndrome after a rubella outbreak. Pediatr Infect Dis J 1995;14:573–78.
- Orenstein WA, Hadler S, Wharton M. Trends in vaccine-preventable diseases. Semin Pediatr Infect Dis 1997;8:23–33
- Reef SE, Frey TK, Theall K, et al. The changing epidemiology of rubella in the 1990s. JAMA 2002;287:464–72.
- Institute of Medicine. 2012. Adverse Events of Vaccines: Evidence and Causality. Washington D.C. : The National Academies Press.
- Page last reviewed: November 15, 2016
- Page last updated: July 30, 2015
- Content source:


 ShareCompartir
ShareCompartir