Tetanus
On this Page
Tetanus is an acute, often fatal, disease caused by an exotoxin produced by the bacterium Clostridium tetani. It is characterized by generalized rigidity and convulsive spasms of skeletal muscles. The muscle stiffness usually involves the jaw (lockjaw) and neck and then becomes generalized.
Tetanus
- Etiology discovered in 1884 by Carle and Rattone
- Passive immunization used for treatment and prophylaxis during World War I
- Tetanus toxoid first widely used during World War II
Although records from antiquity (5th century BCE) contain clinical descriptions of tetanus, it was Carle and Rattone in 1884 who first produced tetanus in animals by injecting them with pus from a fatal human tetanus case. During the same year, Nicolaier produced tetanus in animals by injecting them with samples of soil. In 1889, Kitasato isolated the organism from a human victim, showed that it produced disease when injected into animals, and reported that the toxin could be neutralized by specific antibodies. In 1897, Nocard demonstrated the protective effect of passively transferred antitoxin, and passive immunization in humans was used for treatment and prophylaxis during World War I. A method for inactivating tetanus toxin with formaldehyde was developed by Ramon in the early 1920’s which led to the development of tetanus toxoid by Descombey in 1924. It was first widely used during World War II.
Clostridium tetani
Clostridium tetani
- Anaerobic gram-positive, spore-forming bacteria
- Spores found in soil, animal feces
- Two exotoxins produced with growth of bacteria
- Tetanospasmin estimated human lethal dose = 2.5 ng/kg
C. tetani is a slender, gram-positive, anaerobic rod that may develop a terminal spore, giving it a drumstick appearance. The organism is sensitive to heat and cannot survive in the presence of oxygen. The spores, in contrast, are very resistant to heat and the usual antiseptics. They can survive autoclaving at 249.8°F (121°C) for 10-15 minutes. The spores are also relatively resistant to phenol and other chemical agents.
The spores are widely distributed in soil and in the intestines and feces of horses, sheep, cattle, dogs, cats, rats, guinea pigs, and chickens. Manure-treated soil may contain large numbers of spores. In agricultural areas, a significant number of human adults may harbor the organism. The spores can also be found on skin surfaces and in contaminated heroin.
C. tetani produces two exotoxins, tetanolysin and tetanospasmin. The function of tetanolysin is not known with certainty. Tetanospasmin is a neurotoxin and causes the clinical manifestations of tetanus. On the basis of weight, tetanospasmin is one of the most potent toxins known. The estimated minimum human lethal dose is 2.5 nanograms per kilogram of body weight (a nanogram is one billionth of a gram), or 175 nanograms for a 70-kg (154lb) human.
Pathogenesis
Tetanus Pathogenesis
- Anaerobic conditions allow germination of spores and production of toxins
- Toxin binds in central nervous system
- Interferes with neurotransmitter release to block inhibitor impulses
- Leads to unopposed muscle contraction and spasm
Tetanus Clinical Features
- Incubation period; 8 days (range, 3-21 days)
- Three clinical forms: local (uncommon), cephalic (rare), generalized (most common)
- Generalized tetanus: descending pattern of trismus (lockjaw), stiffness of the neck, difficulty swallowing, rigidity of abdominal muscles
- spasms continue for 3-4 weeks
- complete recovery may take months
Neonatal Tetanus
- Generalized tetanus in newborn infant
- Infant born without protective passive immunity
- 58,000 neonates died in 2010 worldwide
C. tetani usually enters the body through a wound. In the presence of anaerobic (low oxygen) conditions, the spores germinate. Toxins are produced and disseminated via blood and lymphatics. Toxins act at several sites within the central nervous system, including peripheral motor end plates, spinal cord, and brain, and in the sympathetic nervous system. The typical clinical manifestations of tetanus are caused when tetanus toxin interferes with release of neurotransmitters, blocking inhibitor impulses. This leads to unopposed muscle contraction and spasm. Seizures may occur, and the autonomic nervous system may also be affected.
Clinical Features
The incubation period ranges from 3 to 21 days, usually about 8 days. In general the further the injury site is from the central nervous system, the longer is the incubation period. Shorter incubation periods are associated with a higher chance of death. In neonatal tetanus, symptoms usually appear from 4 to 14 days after birth, averaging about 7 days.
On the basis of clinical findings, three different forms of tetanus have been described.
Local tetanus is an uncommon form of the disease, in which patients have persistent contraction of muscles in the same anatomic area as the injury. These contractions may persist for many weeks before gradually subsiding. Local tetanus may precede the onset of generalized tetanus but is generally milder. Only about 1% of cases are fatal.
Cephalic tetanus is a rare form of the disease, occasionally occurring with otitis media (ear infections) in which C. tetani is present in the flora of the middle ear, or following injuries to the head. There is involvement of the cranial nerves, especially in the facial area.
The most common type (about 80%) of reported tetanus is generalized tetanus. The disease usually presents with a descending pattern. The first sign is trismus or lockjaw, followed by stiffness of the neck, difficulty in swallowing, and rigidity of abdominal muscles. Other symptoms include elevated temperature, sweating, elevated blood pressure, and episodic rapid heart rate. Spasms may occur frequently and last for several minutes. Spasms continue for 3-4 weeks. Complete recovery may take months.
Neonatal tetanus (NT) is a form of generalized tetanus that occurs in newborn infants. Neonatal tetanus occurs in infants born without protective passive immunity, because the mother is not immune. It usually occurs through infection of the unhealed umbilical stump, particularly when the stump is cut with an unsterile instrument. Neonatal tetanus is common in some developing countries but very rare in the United States. World Health Organization (WHO) estimates that in 2010, 58,000 newborns died from NT, a 93% reduction from the situation in the late 1980s.
Complications
Tetanus Complications
- Laryngospasm
- Fractures
- Hypertension and/or abnormal heart rhythm
- Nosocomial infections
- Pulmonary embolism
- Aspiration pneumonia
- Death
Laryngospasm (spasm of the vocal cords) and/or spasm of the muscles of respiration leads to interference with breathing. Fractures of the spine or long bones may result from sustained contractions and convulsions. Hyperactivity of the autonomic nervous system may lead to hypertension and/or an abnormal heart rhythm.
Nosocomial infections are common because of prolonged hospitalization. Secondary infections may include sepsis from indwelling catheters, hospital-acquired pneumonias, and decubitus ulcers. Pulmonary embolism is particularly a problem in drug users and elderly patients. Aspiration pneumonia is a common late complication of tetanus, found in 50%-70% of autopsied cases. In recent years, tetanus has been fatal in approximately 11% of reported cases. Cases most likely to be fatal are those occurring in persons 60 years of age and older (18%) and unvaccinated persons (22%). In about 20% of tetanus deaths, no obvious pathology is identified and death is attributed to the direct effects of tetanus toxin.
Laboratory Diagnosis
No laboratory findings are characteristic of tetanus. The diagnosis is entirely clinical and does not depend upon bacteriologic confirmation. C. tetani is recovered from the wound in only 30% of cases and can be isolated from patients who do not have tetanus. Laboratory identification of the organism depends most importantly on the demonstration of toxin production in mice.
Medical Management
All wounds should be cleaned. Necrotic tissue and foreign material should be removed. If tetanic spasms are occurring, supportive therapy and maintenance of an adequate airway are critical.
Tetanus immune globulin (TIG) is recommended for persons with tetanus. TIG can only help remove unbound tetanus toxin. It cannot affect toxin bound to nerve endings. A single intramuscular dose of 500 units is generally recommended for children and adults, with part of the dose infiltrated around the wound if it can be identified. Intravenous immune globulin (IVIG) contains tetanus antitoxin and may be used if TIG is not available.
Because of the extreme potency of the toxin, tetanus disease does not result in tetanus immunity. Active immunization with tetanus toxoid should begin or continue as soon as the person’s condition has stabilized.
Wound Management
Tetanus Wound Management
| Clean, minor wounds | All other wounds* | |||
|---|---|---|---|---|
| Vaccination History | Tdap or Td† | TIG | Tdap or Td† | TIG |
| Unknown or fewer than 3 doses | Yes | No | Yes | Yes |
| 3 or more doses | No§ | No | No¶ | No |
*Such as, but not limited to, wounds contaminated with dirt, feces, soil, and saliva; puncture wounds; avulsions; and wounds resulting from missiles, crushing, burns, and frostbite.
†Tdap is preferred to Td for adults who have never received Tdap. Single antigen tetanus toxoid (TT) is no longer available in the United States.
§Yes, if more than ten years since the last tetanus toxoid-containing vaccine dose.
¶Yes, if more than five years since the last tetanus toxoid-containing vaccine dose.
Tetanus Epidemiology
- Reservoir
- soil and intestine of animals and humans
- Transmission
- contaminated wounds
- tissue injury
- Temporal pattern
- peak in summer or wet season
- Communicability
- not contagious
Antibiotic prophylaxis against tetanus is neither practical nor useful in managing wounds; proper immunization plays the more important role. The need for active immunization, with or without passive immunization, depends on the condition of the wound and the patient’s immunization history (see MMWR 2006;55[RR-17] for details). Rarely have cases of tetanus occurred in persons with a documented primary series of tetanus toxoid.
Persons with wounds that are neither clean nor minor, and who have had fewer than 3 prior doses of tetanus toxoid or have an unknown history of prior doses should receive TIG as well as Td or Tdap. This is because early doses of toxoid may not induce immunity, but only prime the immune system. The TIG provides temporary immunity by directly providing antitoxin. This ensures that protective levels of antitoxin are achieved even if an immune response has not yet occurred.
Epidemiology
Occurrence
Tetanus occurs worldwide but is most frequently encountered in densely populated regions in hot, damp climates with soil rich in organic matter.
Reservoir
Organisms are found primarily in the soil and intestinal tracts of animals and humans.
Mode of Transmission
Transmission is primarily by contaminated wounds (apparent and inapparent). The wound may be major or minor. In recent years, however, a higher proportion of patients had minor wounds, probably because severe wounds are more likely to be properly managed. Tetanus may follow elective surgery, burns, deep puncture wounds, crush wounds, otitis media (ear infections), dental infection, animal bites, abortion, and pregnancy.
Communicability
Tetanus is not contagious from person to person. It is the only vaccine-preventable disease that is infectious but not contagious.
Secular Trends in the United States
Tetanus—United States, 1947-2012
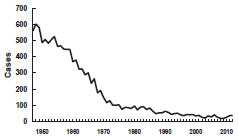
Source: National Notifiable Disease Surveillance System, CDC
Tetanus—United States, 1980-2012
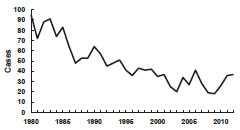
Source: National Notifiable Disease Surveillance System, CDC
Tetanus—United States, 2001-2008 Age Distribution
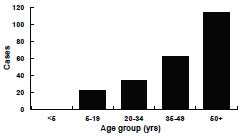
N=233. MMWR 2011;60(No. 12):365-9.
DTaP, DT, Td, and Tdap
| Type | Diphtheria | Tetanus |
|---|---|---|
| DTaP, DT | 6.7-25 Lf units | 5-10 Lf units |
| Td, Tdap (adults) | 2-2.5 Lf units | 2-5 Lf units |
DTaP and pediatric DT used through age 6 years. Adult Td for persons 7 years and older. Tdap for persons 10 years and older (Boostrix) or 10 through 64 years (Adacel)
Tetanus Toxoid
- Formalin-inactivated tetanus toxin
- Schedule
- three or four doses plus booster
- booster every 10 years
- Efficacy
- approximately 100%
- Duration
- approximately 10 years
- Should be administered with diphtheria toxoid as DTaP, DT, Td, or Tdap
A marked decrease in mortality from tetanus occurred from the early 1900s to the late 1940s. In the late 1940s, tetanus toxoid was introduced into routine childhood immunization and tetanus became nationally notifiable. At that time, 500-600 cases (approximately 0.4 cases per 100,000 population) were reported per year.
After the 1940s, reported tetanus incidence rates declined steadily. Since the mid-1970s, 50-100 cases (~0.05 cases per 100,000) have been reported annually. From 2000 through 2007 an average of 31 cases were reported per year. The death-to-case ratio has declined from 30% to approximately 10% in recent years. An all-time low of 18 cases (0.01 cases per 100,000) was reported in 2009.
During 2001 through 2008, the last years for which data have been compiled, a total of 233 tetanus cases were reported, an average of 29 cases per year. Among the 197 cases with known outcomes the case-fatality rate was 13%. Age of onset was reported for all 233 cases, of which, 49% were among persons 50 years of age or older. The median age was 49 years (range 5-94 years). A total of 138 (59%) were male. Incidence was similar among races. The incidence among Hispanics was almost twice that among non-Hispanics. However, when intravenous drug users (IDUs) were excluded the incidence was almost the same among Hispanics and non-Hispanics. Between 18 and 37 cases of tetanus were reported annually in the United States between 2009 and 2012 (average 29 cases per year).
Almost all reported cases of tetanus are in persons who have either never been vaccinated, or who completed a primary series but have not had a booster in the preceding 10 years.
Heroin users, particularly persons who inject themselves subcutaneously, appear to be at high risk for tetanus. Quinine is used to dilute heroin and may support the growth of C. tetani.
Neonatal tetanus is rare in the United States, with only two cases reported since 1989. Neither of the infants’ mothers had ever received tetanus toxoid.
Tetanus toxoid vaccination status was reported for 92 (40%) of the 233 patients. Thirty-seven patients (41%) had never received a tetanus toxoid-containing product, 26 (28%) had received 1 dose, five (5%) had received 3 doses, and 24 (26%) had received 4 or more doses. Seven (24%) of 29 patients with 3 or more doses had received their last dose within the previous 10 years, 18 (62%) between 10 and 54 years previously, and four (14%) reported an unknown interval since their last dose.
Among 195 patients whose medical history was known, 30 (15%) were reported to have diabetes. Twenty-seven (15%) of 176 patients whose status was known were IDUs, of whom 16 (59%) were Hispanic. An acute wound preceded disease onset in 167 (72%) patients. Of those patients’ wounds, 132 (79%) were either punctures or contaminated, infected, or devitalized wounds considered tetanus-prone and eligible to receive TIG. Case reports for 51 (84%) of those who sought care were sufficiently complete to evaluate prophylaxis received; 49 (96%) did not receive appropriate tetanus toxoid prophylaxis or tetanus toxoid plus TIG, as is currently recommended. Among all 233 patients, 31 (13%) reported a chronic wound or infection (e.g., diabetic ulcer or dental abscess) before disease onset. Twenty-two (9%) reported no wounds or infections. Of these, 14 were IDUs.
Tetanus Toxoid
Characteristics
Tetanus toxoid was first produced in 1924, and tetanus toxoid immunizations were used extensively in the armed services during World War II. Tetanus cases among this population declined from 70 in World War I (13.4/100,000 wounds and injuries) to 12 in World War II (0.44/100,000). Of the 12 case-patients, half had received no prior toxoid.
Tetanus toxoid consists of a formaldehyde-treated toxin. The toxoid is standardized for potency in animal tests according to Food and Drug Administration (FDA) regulations. Occasionally, potency is mistakenly equated with Lf units, which are a measure of the quantity of toxoid, not its potency in inducing protection.
There are two types of toxoid available—adsorbed (aluminum salt precipitated) toxoid and fluid toxoid. Although the rates of seroconversion are about equal, the adsorbed toxoid is preferred because the antitoxin response reaches higher titers and is longer lasting than that following the fluid toxoid.
Tetanus toxoid is available combined with diphtheria toxoid as pediatric diphtheria-tetanus toxoid (DT) or adult tetanus-diphtheria (Td), and with both diphtheria toxoid and acellular pertussis vaccine as DTaP or Tdap. Tetanus toxoid is also available as combined DTaP-HepB-IPV (Pediarix) and DTaP-IPV/Hib (Pentacel) —see Pertussis chapter for more information. Pediatric formulations (DT and DTaP) contain a similar amount of tetanus toxoid as adult Td, but contain 3 to 4 times as much diphtheria toxoid. Children younger than 7 years of age should receive either DTaP or pediatric DT. Persons 7 years of age or older should receive the adult formulation (adult Td), even if they have not completed a series of DTaP or pediatric DT. Tetanus toxoid is given in combination with diphtheria toxoid, since periodic boosting is needed for both antigens. Two brands of Tdap are available: Boostrix (approved for persons 10 and older) and Adacel (approved for persons 10 through 64 years of age). DTaP and Tdap vaccines do not contain thimerosal as a preservative.
Routine DTaP Primary Vaccination Schedule
| Dose | Age | Interval |
|---|---|---|
| Primary 1 | 2 months | --- |
| Primary 2 | 4 months | 4 weeks |
| Primary 3 | 6 months | 4 weeks |
| Primary 4 | 15-18 months | 6 months |
Children Who Receive DT
- The number of doses of DT needed to complete the series depends on the child’s age at the first dose:
- if first dose given at younger than 12 months of age, 4 doses are recommended
- if first dose given at 12 months or older, 3 doses complete the primary series
Tetanus, Diphtheria and Pertussis Booster Doses
- 4 through 6 years of age, before entering school (DTaP)
- 11 or 12 years of age (Tdap)
- Every 10 years thereafter (Td)
Immunogenicity and Vaccine Efficacy
After a primary series (three properly spaced doses of tetanus toxoid in persons 7 years of age and older, or four doses in children younger than 7 years of age) essentially all recipients achieve antitoxin levels considerably greater than the protective level of 0.1 IU/mL.
Efficacy of the toxoid has never been studied in a vaccine trial. It can be inferred from protective antitoxin levels that a complete tetanus toxoid series has a clinical efficacy of virtually 100%; cases of tetanus occurring in fully immunized persons whose last dose was within the last 10 years are extremely rare.
Antitoxin levels decrease with time. While some persons may be protected for life, by 10 years after the last dose, most persons have antitoxin levels that only approach the minimal protective level. As a result, routine boosters are recommended every 10 years.
In a small percentage of individuals, antitoxin levels fall below the minimal protective level before 10 years have elapsed. To ensure adequate protective antitoxin levels, persons who sustain a wound that is other than clean and minor should receive a tetanus booster if more than 5 years have elapsed since their last dose. (See Wound Management for details on persons who previously received less than three doses).
Vaccination Schedule and Use
DTaP (diphtheria and tetanus toxoids and acellular pertussis vaccine) is the vaccine of choice for children 6 weeks through 6 years of age. The usual schedule is a primary series of four doses at 2, 4, 6, and 15-18 months of age. The first, second, and third doses of DTaP should be separated by a minimum of 4 weeks. The fourth dose should follow the third dose by no less than 6 months and should not be administered before 12 months of age.
If a child has a valid contraindication to pertussis vaccine, pediatric DT should be used to complete the vaccination series. If the child was younger than 12 months old when the first dose of DT was administered (as DTaP or DT), the child should receive a total of four primary DT doses. If the child was 12 months of age or older at the time that the first dose of DT was administered, three doses (third dose 6-12 months after the second) completes the primary DT series.
If the fourth dose of DTaP, DTP, or DT is administered before the fourth birthday, a booster dose is recommended at 4-6 years of age. The fifth dose is not required if the fourth dose was given on or after the fourth birthday.
Routine Td Schedule Unvaccinated Persons 7 Years of Age or Older
| Dose* | Interval |
|---|---|
| Primary 1 | --- |
| Primary 2 | 4 weeks |
| Primary 3 | 6 to 12 months |
| Booster dose every 10 years |
Because of waning antitoxin titers, most persons have antitoxin levels below the optimal level 10 years after the last dose of DTaP, DTP, DT, or Td. Additional booster doses of tetanus and diphtheria toxoids are required every 10 years to maintain protective antitoxin titers. The first booster dose of Td may be given at 11 or 12 years of age if at least 5 years have elapsed since the last dose of DTaP, DTP, or DT. The Advisory Committee on Immunization Practices (ACIP) recommends that this dose be administered as Tdap. If a dose is given sooner as part of wound management, the next booster is not needed for 10 years thereafter. More frequent boosters are not indicated and have been reported to result in an increased incidence and severity of local adverse reactions.
Td is the vaccine of choice for children 7 years and older and for adults. A primary series is three or four doses, depending on whether the person has received prior doses of diphtheria-containing vaccine and the age these doses were administered. The number of doses recommended for children who received one or more doses of DTP, DTaP, or DT before age 7 years is discussed above. For unvaccinated persons 7 years and older (including persons who cannot document prior vaccination), the primary series is three doses. The first two doses should be separated by at least 4 weeks, and the third dose given 6 to 12 months after the second. ACIP recommends that one of these doses (preferably the first) be administered as Tdap. A booster dose of Td should be given every 10 years. Tdap is approved for a single dose at this time (i.e., it should not be used for all the doses of Td in a previously unvaccinated person 7 years or older). Refer to the Pertussis chapter for more information about Tdap.
Interruption of the recommended schedule or delay of subsequent doses does not reduce the response to the vaccine when the series is finally completed. There is no need to restart a series regardless of the time elapsed between doses.
Tetanus disease does not confer immunity because of the very small amount of toxin required to produce illness. Persons recovering from tetanus should begin or complete active immunization with a tetanus toxoid-containing vaccine during convalescence.
Contraindications and Precautions to Vaccination
Diphtheria and Tetanus Toxoids Contraindications and Precautions
- Severe allergic reaction to vaccine component or following a prior dose
- Moderate or severe acute illness
Tetanus Toxoid Adverse Events
- Institute of Medicine favors a causal relationship
- anaphylaxis
- Institute of Medicine rejects a causal relationship
- type 1 diabetes
- Institute of Medicine finds evidence inadequate to support or reject a causal relationship
- peripheral neuropathy
- Guillain-Barré syndrome (GBS)
Diphtheria and Tetanus Toxoids Adverse Reactions
- Local reactions (erythema, induration) are common
- Fever and systemic symptoms not common
- Exaggerated local reactions (Arthus-type) occasionally reported
- Brachial neuritis
A severe allergic reaction (anaphylaxis) to a vaccine component or following a prior dose of tetanus toxoid is a contraindication to receipt of tetanus toxoid. If a generalized reaction is suspected to represent allergy, it may be useful to refer an individual for appropriate skin testing before discontinuing tetanus toxoid immunization. A moderate or severe acute illness is a precaution to routine vaccination, but a minor illness is not. If moderate to severe acute illness accompanies a wound that is neither clean nor minor, the risk of withholding tetanus-toxoid vaccine outweighs the risk of administering tetanus-toxoid vaccine, so the vaccine should be given as part of wound management.
If a contraindication to using tetanus toxoid-containing preparations exists, passive immunization with tetanus immune globulin (TIG) should be considered whenever an injury other than a clean minor wound is sustained.
See the Pertussis chapter for additional information on contraindications and precautions to Tdap.
Adverse Events Following Vaccination
Severe systemic reactions such as generalized urticaria (hives), anaphylaxis, or neurologic complications have been reported after receipt of tetanus toxoid. A few cases of peripheral neuropathy and Guillain-Barré syndrome (GBS) have been reported following tetanus toxoid vaccine administration. A 2011 Institute of Medicine review found evidence to be inadequate to accept or reject a causal relationship between tetanus toxoid vaccine and peripheral neuropathy and GBS, and favored rejection of a causal relationship between tetanus toxoid and type 1 diabetes, and supported a causal relationship between tetanus toxoid and anaphylaxis.
Adverse Reactions Following Vaccination
Local reactions (e.g., erythema, induration, pain at the injection site) are common but are usually self-limited and require no therapy. A nodule may be palpable at the injection site of adsorbed products for several weeks. Abscess at the site of injection has been reported. Fever and other systemic symptoms are not common.
Exaggerated local (Arthus-like) reactions are not common following receipt of a diphtheria- or tetanus- containing vaccine. These reactions present as extensive painful swelling, often from shoulder to elbow. They generally begin from 2 to 8 hours after injections and are reported most often in adults, particularly those who have received frequent doses of diphtheria or tetanus toxoid. Persons experiencing these severe reactions usually have very high serum antitoxin levels; they should not be given further routine or emergency booster doses of Td more frequently than every 10 years. Less severe local reactions may occur in persons who have multiple prior boosters.
In 1994 the Institute of Medicine concluded that the available evidence favors a causal relationship between tetanus toxoid and brachial neuritis in the 1 month after immunization at a rate of 0.5 to 1 case per 100,000 toxoid recipients.
Vaccine Storage and Handling
All tetanus-toxoid containing vaccines should be maintained at refrigerator temperature between 35°F and 46°F (2°C and 8°C). Manufacturer package inserts contain additional information. For complete information on best practices and recommendations please refer to CDC’s Vaccine Storage and Handling Toolkit [4.33 MB, 109 pages].
Acknowledgment
The editors thank Drs. Gina Mootrey, Tejpratap Tiwari, and Cindy Weinbaum, CDC for their assistance in updating this chapter.
Selected References
- CDC. Diphtheria, tetanus, and pertussis: Recommendations for vaccine use and other preventive measures. Recommendations of the Immunization Practices Advisory Committee (ACIP). MMWR 1991;40(No. RR-10):1-28.
- CDC. Pertussis vaccination: use of acellular pertussis vaccines among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 1997;46(No. RR-7):1-25.
- CDC. Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2006;55(No. RR-3):1-34.
- CDC. Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines. Recommendations of the Advisory Committee on Immunization Practices (ACIP) and Recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC), for Use of Tdap Among Health-Care Personnel. MMWR 2006;55(No. RR-17):1-33.
- CDC. Tetanus surveillance—United States, 1998-2000. MMWR 2003;52(No. SS-3):1-12.
- CDC. Updated Recommendations for Use of Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis (Tdap) Vaccine from the Advisory Committee on Immunization Practices, 2011. MMWR 2011;60(No.1):13-15.
- IOM (Institute of Medicine) 2011. Adverse Effects of Vaccines: Evidence and Causality. Washington DC:The National Academies Press.
- Wassilak SGF, Roper MH, Kretsinger K, Orenstein WA. Tetanus toxoid. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. 5th ed. China: Saunders, 2008:805-39.
- World Health Organization. The “high-risk” approach: the WHO-recommended strategy to accelerate elimination of neonatal tetanus. Wlky Epidemiol Rec 1996;71:33-36.
- Page last reviewed: November 15, 2016
- Page last updated: September 8, 2015
- Content source:


 ShareCompartir
ShareCompartir