Rabies Serology
Laboratory testing of human and animal sera is used to evaluate an immune response to rabies virus antigens. The below information on rabies virus serology should help to alleviate concerns and misunderstanding based, in part, upon responses to questions frequently received by CDC.
Who should receive routine rabies virus serological testing?
Typically, rabies virus neutralizing antibody tests, such as the rapid fluorescent focus inhibition test, are used to monitor antibody levels in persons that may have an occupational risk of rabies virus exposure (e.g. veterinarians, rabies virus laboratory workers, etc.). In some cases, such serological testing is used to check the immune response of a person undergoing rabies postexposure prophylaxis when major deviations in the vaccination schedule occur, or there are concerns about a patient’s immune status. In previous studies, all healthy persons tested 2-4 weeks after completion of pre-exposure and postexposure rabies virus prophylaxis, in accordance with the United States Advisory Committee on Immunization Practices (ACIP) guidelines, demonstrated a significant antibody response to rabies virus.
For most persons, completing pre-exposure or postexposure prophylaxis routine serological testing is not necessary to document seroconversion, unless the:
- the person is immunosuppressed;
- significant deviations of the prophylaxis schedule have occurred;
- the patient initiated vaccination internationally with a product of questionable quality; or
- the person’s antibody status is being monitored routinely due to occupational exposure to rabies virus.
What is the Rapid Fluorescent Focus Inhibition Test (RFFIT)?
The RFFIT is a rabies virus neutralization test performed in cell culture to determine the rabies virus neutralizing antibody level in human or animal sera. Immunofluorescent staining of infected cells is used as an indicator of rabies virus replication. The RFFIT takes ~20 hours and is both sensitive and specific in the hands of well-trained laboratory personnel.
While rabies virus antibodies can be measured through the application of an Enzyme-Linked Immunosorbant Assay (ELISA), these tests measure binding antibodies to viral antigens rather than functional neutralizing antibodies measured in the RFFIT. In some cases, ELISA tests can provide false positive results if they detect antibodies that bind to rabies virus, but are unable to neutralize the virus. The RFFIT is the current gold standard serological assay recommended by both the Advisory Committee on Immunization Practices (ACIP) and the World Health Organization (WHO). Other serological tests, such as an ELISA, are more appropriate for research, and are not recommended for samples requiring clinical decision making by clinicians based upon current ACIP and WHO recommendations.
How is the Rapid Fluorescent Focus Inhibition Test (RFFIT) conducted?
The RFFIT is performed by mixing different dilutions of test sera (animal or human) with a constant amount of rabies virus in a multi-chambered slide. The serum-virus mixture is incubated for a short time to allow time for any antibodies in the serum to neutralize rabies virus, before adding the mixture to cells, in which the virus can replicate. The samples are incubated for ~20 hours to allow any non-neutralized virus to replicate in the cells before they are fixed and stained to detect any rabies virus production, by reading the slide using a fluorescent microscope.
In the RFFIT, a total of 20 microscopic fields are read for each serum dilution, and compared against a control slide containing reference serum and virus dilutions. The number of infected fields for each serum dilution is used to determine the rabies virus neutralizing titer.
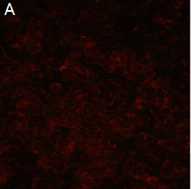
Figure A: An example of a microscopic field where virus has been neutralized.
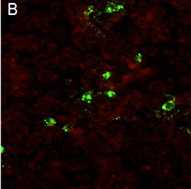
Figure B: An example of a microscopic field where virus was not neutralized.
How are serum neutralization end-point titers and international units calculated?
Serum neutralization end-point titers and international units are calculated based on the number of infected fields read for each serum dilution.
Determining the 50% end-point titers of serum:
The serum neutralization end-point titer, the dilution factor of the highest serum dilution in which there is a 50% reduction in the number of fluorescing foci, can be determined by the number of fluorescing foci at each dilution. The Reed-Meunch formula is applied to calculate the difference between the logarithm of the starting dilution and the logarithm of the 50% end-point dilution.
The Reed-Meunch formula:

An example of calculated (Reed-Meunch Method) 50% serum end-point titers corresponding to the number of infected fields observed for different serum dilutions.
| Serum dilution | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1:5 | 11 | 11 | 10 | 9 | 9 | 8 | 7 | 7 | 6 | 5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 |
| 1:25 | 56 | 54 | 50 | 50 | 45 | 42 | 40 | 36 | 33 | 29 | 25 | 21 | 19 | 17 | 16 | 14 | 13 | 13 | 13 | 12 | 11 |
| 1:125 | 280 | 270 | 250 | 250 | 230 | 210 | 200 | 180 | 170 | 145 | 125 | 110 | 95 | 85 | 75 | 70 | 70 | 65 | 60 | 60 | 56 |
Conversions of antibody titer to IU/mL
The results of the RFFIT can be expressed as a serum titer or in International Units (IU) of antibody per milliliter of serum. In either case, a reference serum of known titer is required as a control. When the results are to be reported as a titer, the reference serum is used as a control to ensure the test was appropriately conducted.
When the test serum results are expressed in IU, neutralizing activity of the test serum is compared to that of a reference serum with a known IU concentration. Typically, the reference serum is diluted to contain 2 IU/ml and tested with the test serum. The 50% end-point titers of the reference serum and the test serum are then compared in the following formula for calculation of IU/ml in the test serum:
End-point titer of the reference

Example:
Test serum titer = 54 (1 infected field at a serum dilution of 1:25)
Reference serum titer = 200 (6 infected fields at a serum dilution of 1:125)
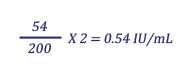
What does a rabies virus titer mean?
A rabies antibody titer is essentially an estimation of an immune response against rabies virus (either through exposure or vaccination). The RFFIT is one method which provides a laboratory measurement of the ability of an individual human or animal serum sample to neutralize rabies virus.
There is no “protective” titer against rabies virus. In animal studies, survival against rabies virus infection is often more likely to occur the higher an animal’s titer at time of infection, but not a definite indicator of survival. For example in one study of orally vaccinated raccoons 39% of animals with no detectable titer at infection (<0.05 IU/mL) survived, compared to 90% of animals with a titer between 0.05-0.49 and 100% of animals with a titer >0.5 IU/mL. Mounting a rapid antibody response (referred to as an anamnestic response) is often a better indicator of surviving exposure which is one reason additional doses of vaccine are recommended after an exposure, to ensure a rapid antibody response, even if a person has been previously vaccinated.
What ‘cut off’ titer should be used to define adequate immunization?
The ACIP recommends complete neutralization of rabies virus at a serum dilution of 1:5 as minimum evidence of circulating rabies virus neutralizing antibodies. Interpreting the titer depends on the clinical situation in which the test was requested.
Current ACIP recommendations outline the frequency of titer checks for persons with an occupational risk of rabies virus exposure. Generally, antibody levels are expected to be highest approximately 2-3 weeks after completing a primary rabies virus vaccination series. After being vaccinated, antibody levels subside over time. Complete neutralization of rabies virus at a serum dilution of 1:5 (~0.11 IU/mL) is recommended by the ACIP as evidence that an individual still has a detectable level of rabies virus neutralizing antibodies. At this level, an immune competent individual would be expected to mount a rapid response to a booster dose of rabies vaccine in the event of an exposure, precluding the need of rabies immune globulin during postexposure prophylaxis. If a person with an occupational risk of rabies virus exposure does not have evidence of rabies virus neutralizing antibodies at a serum dilution of at least 1:5 (~0.11 IU/mL), then they should receive a single booster dose of rabies vaccine.
Evaluating the immune response of individuals receiving prophylaxis after a potential rabies virus exposure should consider the reason a titer is being requested (i.e. deviation from schedule or questions about a patient’s immune status). Concerns about a patient’s immune status while receiving rabies virus vaccination may require multiple serum samples to follow their immune response. Previous clinical studies have found that rabies virus neutralizing antibody titers range between ~6-12 IU/mL, among healthy persons completing the vaccination series. When evaluating a patient’s titer during the course of receiving rabies virus vaccination, it is important to consider that a relatively high titer is expected. If questions arise regarding rabies virus prophylaxis, serological testing, antibody levels, and patient response, input should be sought from local, state, or federal public health departments in regards to continued monitoring and management.
Where can I request rabies virus serology using the Rapid Fluorescent Focus Inhibition Test (RFFIT)?
Currently, the RFFIT may be available in a state through a state health department or University. The state or local health department may provide assistance with obtaining serological testing, if available. In addition, two commercial laboratories perform the RFFIT for rabies virus antibody testing.
Atlanta Health Associates*
309 Pirckle Ferry Road, Suite D300
Cumming, GA 30040
Phone: 770-205-9091 or 800-717-5612
Fax: 770-204-9021
www.atlantahealth.net
Kansas State University*
1800 Denison Avenue
Manhattan, KS 66506-5600
Phone: 785-532-4483
http://www.ksvdl.org/rabies-laboratory/
Testing at KSU may also be requested through Quest Labs as Rabies Vaccine Response End Point Titer (order # 5789).
*Use of trade names, commercial sources or private organizations is for identification only and does not imply endorsement by the U.S. Department of Health and Human Services and/or CDC
References and Additional Reading
Briggs DJ, Schwnke JR. Longevity of rabies antibody titer in recipents of human diploid cell rabies vaccine. Vaccine. 1992; 10: 125-9 [ pubmed ]
Smith JS, Yager PA, Baer GM. A rapid reproducible test for determining rabies neutralizing antibody. Bull World Health Org. 1973; 48: 535-541 [ pubmed ]
Related Links
- Page last reviewed: April 15, 2016
- Page last updated: April 15, 2016
- Content source:





 ShareCompartir
ShareCompartir